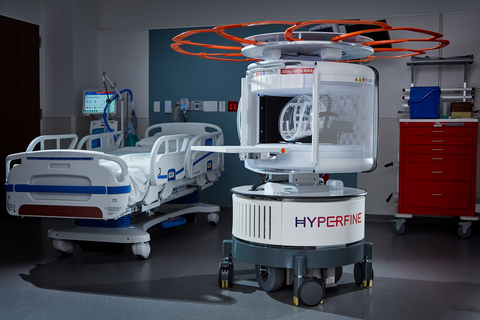
GUILFORD, Conn.– Hyperfine Inc. today announced results of a study of the company’s FDA-cleared portable magnetic resonance imaging (MRI) device, Swoop™, published in Nature Communications. The study, which was conducted at Yale New Haven Hospital, demonstrated high accuracy for Swoop for the detection of hemorrhagic stroke.
“Rapid determination of stroke etiology is absolutely critical to successful treatment and ensuring optimal clinical outcomes for patients,” said Kevin Sheth, M.D., Vice Chair, Clinical and Translational Research, Departments of Neurology and Neurosurgery at the Yale School of Medicine, who served as principal investigator of the study. “This study validates Swoop as an accurate method to detect and characterize intracerebral hemorrhage. The results are exciting because Swoop is readily accessible, providing clinicians with an entirely new option for rapid and convenient assessment of patients with brain injury—which will be particularly useful for settings in which CT and MRI are not readily available, such as intensive care units.”
American Heart Association (AHA) guidelines for stroke management advise that all patients receive rapid brain imaging on hospital arrival to rule out the presence of blood, which is contraindicated for thrombolytic (“clot busting”) drugs. Computed tomography (CT) has been the imaging method of choice for diagnosing hemorrhagic stroke, but growing evidence has shown that MRI is as accurate as CT for detecting acute brain hemorrhage, and avoids the radiation exposure associated with CT.
As the world’s first FDA-cleared bedside MRI system, Hyperfine’s portable Swoop system is designed to allow physicians to rapidly understand the current state of injury to make life-saving decisions. Within minutes, the technology can acquire critical images via a wireless tablet, powered by a standard wall outlet at the patient’s bedside. Because of Swoop’s portability and magnet design, care teams and loved ones can safely stay by the patient’s side during the scan, reducing patient anxiety and providing a more comfortable experience.
“In addition to being time- and resource-intensive, neuroimaging of patients with critical conditions such as brain hemorrhage has unique challenges, including their transport outside the ICU environment and the time spent in less-controlled imaging rooms,” said Murat Günel, M.D., Professor of Neurosurgery and Professor of Genetics and of Neuroscience; Chair, Department of Neurosurgery; and Chief, Neurosurgery, for Yale New Haven Health System. “In addition, transporting patients to an MRI machine poses a potential risk to patients in critical condition due to the occurrence of potential adverse events during transfer. These results show that Hyperfine’s alternative modality may offer a breakthrough for settings in which specialized infrastructure and highly trained technicians are not readily available.”
In the study, critically ill patients were imaged using conventional neuroimaging, either non-contrast CT or conventional MRI, and the Swoop portable MRI system. A total of 144 Swoop examinations were evaluated.
- Blood-negative cases were correctly identified in 85 of 88 cases (96.6% specificity).
- The study found that Swoop correctly detected intracerebral hemorrhage in 45 of 56 cases (80.4% sensitivity).
- To account for confounding effects due to evolving improvements in scanner software and hardware, an identical analysis was performed in two subsets by grouping exams by software versions into the first half of the study and the second half of the study.
- Exams collected during the second half of scanner software versions were correctly classified in 76 of 84 cases (90.5% overall accuracy). Intracerebral hemorrhage was identified in 29 of 34 cases (85.3% sensitivity).
- The study also showed that blood volumes estimated by Swoop correlated with conventional imaging volumes.
- In addition, Swoop-acquired intracerebral hemorrhage characteristics (blood volume) were associated with clinical outcomes.
“The results of this study, completed with our first-generation device and algorithms, are exciting real-world clinical validation of our technology, which we expect will only improve with our advancements in deep learning software currently under review by the FDA,” said Dave Scott, Hyperfine president and CEO. “We are very pleased with the clinical response from our U.S. launch so far, and look forward to accelerating commercial expansion in the U.S. and providing greater access to Swoop innovation globally.”
In January 2021, Hyperfine received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its deep-learning image analysis software, incorporating advanced artificial intelligence (AI) to measure brain structure and pathology. The company has also submitted a new 510(k) application to the FDA for its planned incorporation of deep learning-based image reconstruction.