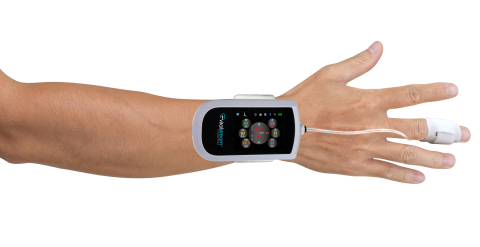
CHARLOTTESVILLE, Va.– Caretaker Medical, a pioneer in wireless “beat by beat” continuous, non-invasive, advanced patient monitoring, today announced U.S. Food and Drug Administration (FDA) clearance of the company’s next-generation VitalStream™ wireless blood pressure and hemodynamic monitoring platform.
Continuous monitoring of blood pressure is of great importance to detect and treat dynamic changes. Traditionally, blood pressure is measured intermittently with traditional cuffs that miss dangerous blood pressure changes, or it is measured continuously with an invasive arterial line, which is costly, requires a skilled clinician, and is difficult to use. VitalStream was designed to eliminate monitoring gaps associated with intermittent spot-check hemodynamic and vital sign measurements.
The VitalStream wireless platform expands the company’s clinically validated PDA™ waveform analysis technology and AI algorithms to measure blood pressure and hemodynamic parameters with every heartbeat, providing an uninterrupted stream of real-time data that eliminates “blind spots” between manual spot-check measurements, without limiting patient mobility.
“Accurate, non-invasive hemodynamic monitoring is certainly preferred to invasive A-lines in most patient populations. My use of the VitalStream monitor in the OR demonstrates compelling benefits in patient comfort, simplicity of use, and continuous, accurate, actionable data,” said Karen Boretsky, M.D., assistant professor of anesthesia at Harvard Medical School and senior associate in perioperative anesthesia, critical care, and pain medicine.
Utilizing a low-pressure finger sensor, VitalStream is a non-invasive alternative to A-lines and arterial catheters, enabling ICU-grade patient monitoring at all points of care to provide clinicians with streaming patient data for early indications of patient deterioration, faster life-saving interventions, and better treatment decisions. VitalStreams’s hemodynamics, waveforms, and vital sign data can be streamed remotely to the company’s mobile app or secure cloud portal, or integrated into other monitoring systems and EMRs with their FDA-cleared software interface SDKs.
“Intraoperative hypotension is common during noncardiac surgery and associated with 30-day major adverse events,” said Ashish Khanna, M.D., associate professor, vice chair for Research, Department of Anesthesiology, Section on Critical Care Medicine at Wake Forest School of Medicine Center. “The ability to noninvasively see beat-by-beat blood pressure and hemodynamic deterioration in real time allows us to make early interventions and potentially improve outcomes.”
More than 150 clinical customers throughout the world have been using Caretaker Medical’s previous generation beat-by-beat blood pressure and vital signs monitor across the full continuum of care, including OR, ICU, ER, COVID-19, Infusion, Sleep Lab, NeuroPsych, Pharma Drug Trials, and remote Hospital-at-Home.
“This is our fourth FDA Clearance expanding our wireless platform and enhancing our core continuous waveform technology and artificial intelligence algorithms,” said Jeff Pompeo, CEO, Caretaker Medical. “VitalStream sets a new standard in beat-by-beat hemodynamics in terms of accuracy, cost, simplicity of use, patient comfort, and streamlined workflow. We’re delivering ICU-grade monitoring that surpasses the most stringent regulatory certifications and clinical accuracy validation requirements to help improve outcomes at all points of care.”
This next-generation VitalStream platform expands on the company’s previous FDA-cleared Caretaker4 wireless patient monitor and PDA and AI algorithms by adding advanced hemodynamics, automated NEWS/MEWS early warning scores, higher resolution data and waveforms, ultra-fast 30-second auto-calibration and setup time, streamlined clinical workflow, more data connectivity options and a smaller wearable form factor.
The VitalStream Wireless Hemodynamic Monitor is available immediately in the United States directly from the company and its distribution partners, and will be available in Europe and Asia next year.