LOS ANGELES– Pearl, the global leader in dental AI solutions, today announced that Second Opinion®, its chairside clinical artificial intelligence (AI) software, has received clearance from the South African Health Products Regulatory Authority (SAHPRA). Second Opinion® is the first and only FDA-cleared chairside AI software to help dentists detect numerous conditions in x-rays of dental patients 12 and older. South Africa joins the growing list of medical device authorizations for Second Opinion®, which already includes clearances in the United States, European Union, Canada, Australia, New Zealand, United Arab Emirates, Saudi Arabia, and Brazil.
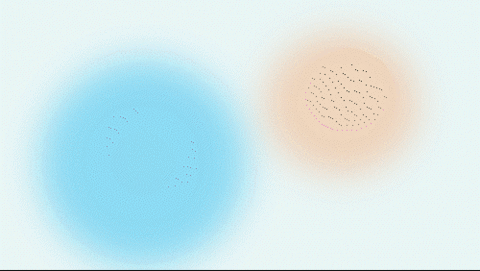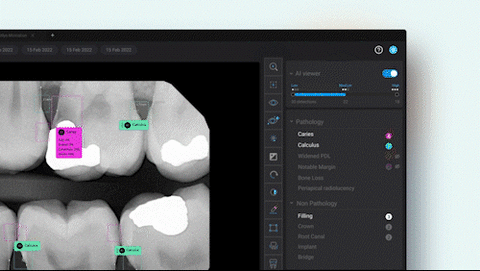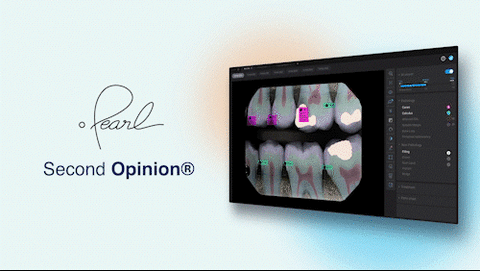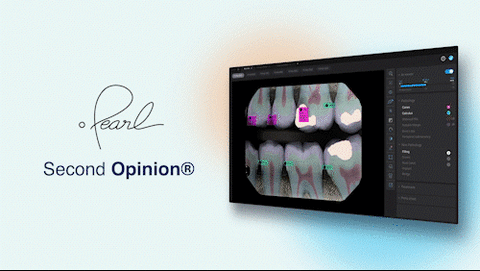
Second Opinion® supports clinicians by applying computer vision technology to patient x-rays to detect a broad range of common dental conditions, including dental caries (cavities), bone loss, root abscesses, calculus (tartar), faulty restorations, impactions, crowns, fillings, root canals, implants and more. It delivers findings in real-time for patient-facing display in the dental operatory, helping dentists ensure the accuracy of their x-ray evaluations and enabling them to better communicate diagnoses to patients.
“We’re excited to bring to South African dentistry the clinical benefits that Second Opinion® is already delivering in so many other countries,” said Ophir Tanz, CEO and founder of Pearl. “Every dental practice in the world wants to deliver care with the greatest consistency, confidence and precision and every dental patient wants to know that the care they receive meets that exceptional standard. Second Opinion® satisfies both of those desires, so its SAHPRA clearance is something that patients and practices in South Africa can smile about.”

