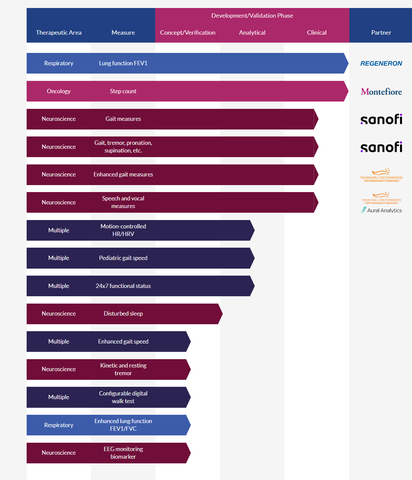
NEW YORK– Koneksa, a healthcare technology company developing evidence-based validated digital biomarkers, today announced the launch of its clinical pipeline featuring biomarkers across neuroscience, oncology, respiratory, and other therapeutic areas. With 15 digital biomarker programs in development, the company will also be launching several upcoming clinical studies. Koneksa and collaborators have published more than 20 peer-reviewed papers.
Koneksa’s digital biomarker platform uses algorithms to analyze and integrate real-time patient data into clinical trials, translating typically analog processes into digital. By developing a suite of validated, evidence-based digital biomarker solutions that can be seamlessly integrated into clinical studies, from at-home spirometry, actigraphy, gait and balance-monitoring tools, to vital-sign monitoring, Koneksa’s biomarker technology potentially increases precision and captures a richer dataset.
The launch of the clinical pipeline will enable Koneksa to expand biomarker evidence generation in the near-term and develop new digital biomarkers to support long-term industry and patient needs. The increasing adoption of clinically-validated digital biomarkers in studies which can be used in an at-home setting, significantly reduces patient burden and increases clinical trial efficiency. Digital biomarkers can also integrate data from multiple sources to help researchers better understand key drivers of disease progression to bring novel medicines to patients faster.
“Digital biomarkers need to be clinically validated. Without that validation, these tools are ultimately not useful in clinical trials,” said Chris Benko, CEO & Founder of Koneksa. “Think of digital biomarkers versus a clinic-based assessment as the difference between a still photo and a video. A still photo is great, but a video can tell a much richer story. Over the last few years, our team has conducted over a dozen analytical studies, with the same level of clinical rigor to validate our digital biomarkers on par with traditional biomarkers for use in clinical trials.”
“The more high quality measurements we capture, the higher precision and more power a clinical trial can have,” said John Wagner, M.D., Ph.D., Chief Medical Officer, Koneksa. “Validation studies have shown that our digital biomarkers are equivalent to or better than the current standard – and we have done that work to the level of rigor required by regulators and the world’s leading biopharmaceutical companies. With the launch of our clinical pipeline, we’re extending our evidence generation and potential new digital biomarkers, further demonstrating that at-home digital biomarkers increase patient access outside of the clinic.”
Koneksa has partnerships with life sciences companies, patient advocacy organizations and academic institutions on its digital biomarker pipeline. In respiratory disease, Koneksa partnered with Regeneron to implement and validate mobile spirometry. In oncology, Koneksa developed a step count measurement, in partnership with Montefiore Medical Center, to better characterize functional status. In neuroscience, the company partnered with Sanofi to develop biomarkers to measure gait in patients with neurological diseases such as multiple sclerosis. Koneksa recently announced a partnership with Aural Analytics to support clinical trials using speech measures to develop a variety of digital biomarkers for Parkinson’s Disease in collaboration with Northwestern University and the Michael J. Fox Foundation.
Koneksa’s development and validation process for its digital biomarker solutions is based on the same FDA guidelines and level of rigor for traditional biomarkers such as the Biomarker Qualification Evidentiary Framework as well as the Digital Health Technologies Remote Data Acquisition In Clinical Investigations draft guidance.