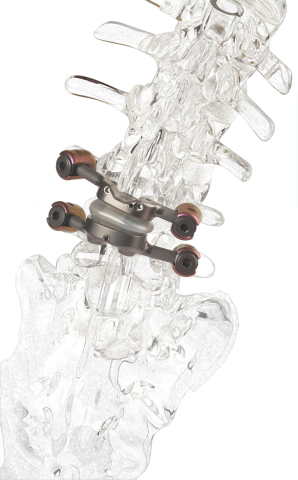
NORWALK, Conn.– Premia Spine, a medical technology company changing the way debilitating chronic leg and back pain is treated, today announced publication of one-year outcomes from the TOPS facet replacement system’s clinical trial in the journal Clinical Spine Surgery. The study, “A Prospective Study of Lumbar Facet Arthroplasty in the Treatment of degenerative Spondylolisthesis and Stenosis: Results from the Total Posterior Spine System (TOPS) IDE Study,” found lumbar facet arthroplasty with the TOPS device demonstrated a statistically significant improvement in all patient-reported outcome measures, a low surgical complication rate, and the ability to maintain motion at the index level while limiting sagittal translation.
The objective of the prospective, randomized, multi-center U.S. Food and Drug Administration (FDA) investigational device exemption (IDE) clinical trial is to compare the two-year clinical and radiographic outcomes and safety profile of patients undergoing either a lumbar TOPS facet arthroplasty or a transforaminal lumbar interbody fusion (TLIF). This publication is the first analysis of early clinical outcomes and complications from the TOPS IDE trial. The study will help surgeons more accurately counsel patients on the best treatment for lumbar spinal stenosis and degenerative spondylolisthesis.
“The one-year data from this study indicate the TOPS System compares favorably in all respects to outcomes reported in the literature after single-level TLIF,” said Dr. Zachariah Pinter, the lead author of the publication. “If at the conclusion of the ongoing clinical trial we see continued favorable outcomes, that would indicate that motion preservation with TOPS facet arthroplasty is a viable alternative to fusion for restoring segmental stability while maintaining motion at the affected segment.”
In the study, 153 patients were enrolled in the investigational arm of the clinical trial and underwent implantation of the TOPS device, with 145 TOPS devices implanted at L4-5 (94.8%), and eight devices implanted at L3-4 (5.2%). Sixty-nine percent (105 patients) of the 153 subjects reached their one-year follow-up at the time of this interim assessment, and were included in the PROMs and radiographic analyses.
Lumbar facet arthroplasty with the TOPS device demonstrated a low complication rate, significant improvement in all PROMs, and the ability to maintain motion at the index level while limiting sagittal translation. The complication rates and improved postoperative PROMs are similar to those reported in the literature following single-level TLIF. No patient in the TOPS cohort required reoperation within the first year owing to adjacent segment degeneration.
“We are encouraged by the data presented in this publication, which show promising results for TOPS when compared to TLIF,” said Ron Sacher, CEO, Premia Spine. “Unlike standard fusion constructs, the purpose of the TOPS device is to maintain motion at a previously pathologic intervertebral segment. These data indicate we are getting closer to our goal of providing a viable alternative to fusion for those suffering from lumbar spinal stenosis and degenerative spondylolisthesis.”
The first and only facet joint replacement system for the lumbar spine, TOPS was developed to provide mobility, stability and durability after decompression for patients with lumbar spinal stenosis and degenerative spondylolisthesis. Spinal stenosis and degenerative spondylolisthesis are painful, debilitating and highly prevalent conditions impacting over 100 million people globally1. An estimated 350,000 people undergo lumbar spinal fusion each year for these conditions2, representing a $2 billion annual addressable global market. TOPS won Breakthrough Device Designation from the US Food and Drug Administration (FDA) in 2021.