ROOT, Switzerland– Novocure (NASDAQ: NVCR) today announced that Health Canada has approved Optune® for the treatment of newly diagnosed and recurrent glioblastoma (GBM). GBM is the most common and one of the most aggressive forms of primary brain cancer. Optune is the first treatment for glioblastoma approved in Canada in over 12 years.
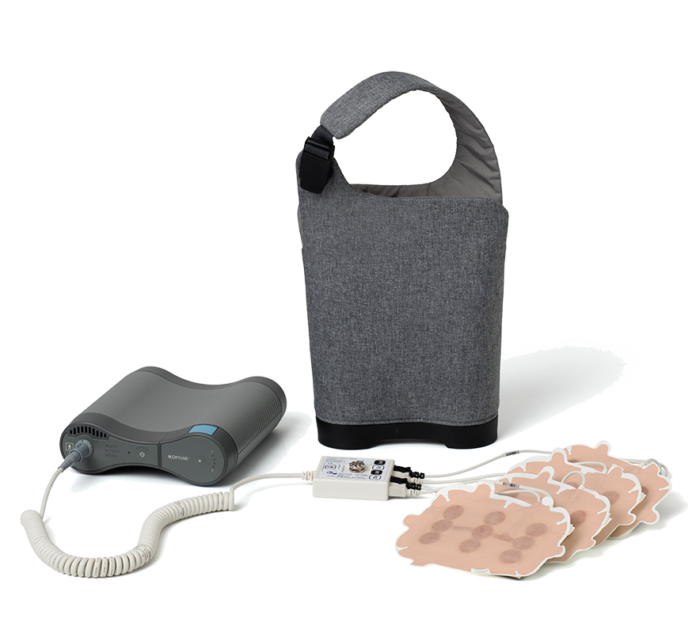
Optune is a medical device that works by creating Tumor Treating Fields (TTFields), which are electric fields that disrupt cancer cell division. Optune delivers TTFields therapy to the region of the tumor. Optune is small and light, weighing 2.7 pounds. This makes Optune wearable and portable, so continuous treatment can be received almost anywhere. Globally, more than 25,000 patients have been treated with Optune, to date.
“We’re very happy that Health Canada has approved Optune for the treatment of GBM,” said Jovan Antunovic, Country Manager of Novocure Canada. “We are grateful for the rapid and diligent review of our submission and for Health Canada’s approval of Optune. This is an important achievement in order to bring our therapy to more patients throughout Canada who can benefit.”
“It is well established that glioblastoma treatment is a high unmet medical need,” said Dr. David Roberge, Professor of Radiology, Radiation Oncology & Nuclear at the CHUM Research Centre and Future President of the Canadian Association of Radiation Oncology. “Patients with GBM have very few effective therapeutic options. The approval of Optune can offer a real benefit to Canadian patients.”
Each year in Canada, 1,600 people are diagnosed with GBM. Novocure’s phase 3 pivotal EF-14 trial compared Optune plus temozolomide to temozolomide alone in 695 patients with newly diagnosed GBM. Patients treated with Optune plus temozolomide experienced overall survival of 20.9 months versus 16 months for patients treated with temozolomide alone (HR, 0.63; 95 percent Cl, 0.53-0.76; P<.001). Optune was safely used together with temozolomide with no significant increase in serious adverse events compared with temozolomide alone. The most common side effect related to Optune was mild to moderate skin irritation. Optune is commercially available as a treatment for GBM in multiple countries in North America, Europe and Asia.
“With Health Canada’s approval of Optune, the Brain Tumour Foundation of Canada (BTFC) is excited that another treatment option will soon be available for patients with GBM,” said Shannon LaHay, CEO of BTFC. “There are many GBM patients and their caregivers looking forward to the availability of Optune in Canada.”
The next critical milestone will be securing reimbursement. Novocure is actively working on this important step in order to ensure that patients have access to Optune for GBM across Canada.
“We are working to ensure that both public and private payers provide access and full reimbursement for Optune as soon as possible,” said Pritesh Shah, Novocure’s Chief Commercial Officer. “We are proud to have reached this milestone as a company and are committed to making our therapy available to all the patients who may benefit throughout the world.”