GOLDEN, Colo.– In a review article1 recently published in Vaccines Journal (MDPI), Dr. Carmen Ledesma-Feliciano, Scientific Affairs Manager at PharmaJet, referencing over 25 studies, discusses multiple injection delivery methods including PharmaJet’s needle-free precision delivery systems, which enhance the clinical performance of DNA-based vaccines.
The COVID-19 pandemic increased visibility of nucleic acid vaccines, such as messenger RNA (mRNA), that provide scalability and can be quickly adapted as new variants appear. DNA vaccines are also nucleic acid vaccines. Currently, 250 vaccine programs2 are invested in DNA vaccines and therapeutics because of their potential benefits.
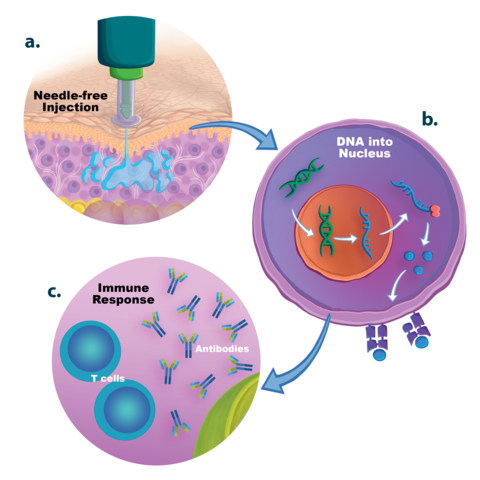
DNA vaccines have inherent advantages compared to other vaccine types, including safety, rapid design and construction, ease and speed to manufacture, and thermostability. However, a major drawback of candidate DNA vaccines delivered by needle-syringe is the inadequate immunogenicity associated with inefficient cellular uptake of the DNA. Multiple techniques have been employed to boost the immunogenicity and protective efficacy of DNA vaccines including physical, electrical, and chemical delivery methods, molecular and traditional adjuvants, and genetic sequence enhancements. Historical limitations can be addressed with new administration technologies such as modern needle-free precision delivery systems.
In studies comparing the PharmaJet precision delivery devices with traditional needle-syringe, Tropis® and Stratis® provided a wider dispersion pattern of the injectate within tissues and more efficient delivery into cells in the case of DNA vaccines. “Delivery of our vaccines with the Stratis device generated better antibody responses as compared to needle-syringe injection. In addition, we found Stratis easier to use, and we observed less discomfort to the laboratory animals,” noted Dr. Ros Chapman, Senior Research Officer, Institute of Infectious Disease and Molecular Medicine, University of Cape Town.
In 2021, PharmaJet’s Tropis was selected as the exclusive delivery system for the first DNA vaccine approved for administration in humans when Zydus Lifesciences obtained Emergency Use Authorization approval from the Drug Controller General of India for its COVID-19 vaccine, ZyCoV-D. Zydus chose Tropis over traditional delivery methods based on pre-clinical and clinical study results that indicated Tropis increased the effectiveness of their vaccine compared to needle-syringe. Several DNA candidate vaccines in combination with PharmaJet devices are in development including for a COVID-19 bivalent booster3, HIV, Zika, influenza, poxvirus, hantavirus, and dengue infections.
“We are enthused to see that pre-clinical results have translated into more effective clinical responses in human trials,” said Chris Cappello, President and CEO of PharmaJet. “We currently have eighty-three studies ongoing with novel pharmaceuticals focusing on nucleic acid technology in addition to other new vaccine technology platforms. We believe that our precision delivery devices have the potential to increase the performance and therefore probability of regulatory success of many of these candidate vaccines.”

