LOS ANGELES– Non-invasive cardiac assessment company Sensydia announced today that it has completed its 225-subject development study at the University of Pittsburgh Medical Center (UPMC). This study was conducted at UPMC to collect data for its innovative AI-powered, non-invasive Cardiac Performance System (CPS™) that uses heart sound analysis to enable earlier detection and more effective therapy guidance for patients suffering from heart failure and pulmonary hypertension.
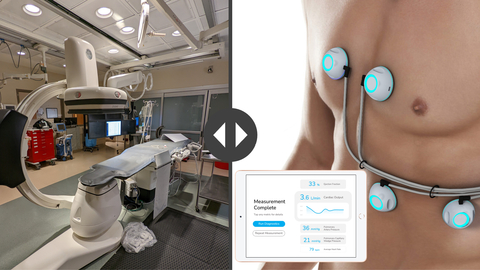
CPS uses ultra-sensitive biosensors to provide clinicians with rapid, non-invasive measurement of ejection fraction, cardiac output, pulmonary artery pressure, and pulmonary capillary wedge pressure in a handheld device. Knowledge of these parameters is critical in the care of individuals with compromised heart function. To obtain these measurements today, patients must undergo echocardiography and invasive catheterization, which are resource intensive and carry significant risk of complications. In contrast, CPS measurements are fast and safe, and can be performed almost anywhere with minimal training.
“When we began this study at UPMC during the height of the COVID-19 pandemic, our original enrollment target for the study was 110 subjects, but we ended up doubling that after hearing positive feedback from the UPMC study staff,” said Anthony Arnold, President and CEO of Sensydia. “This is Sensydia’s fourth successful study and we will continue to collect data across multiple sites to improve the performance and utility of the artificial intelligence algorithms that power our breakthrough CPS platform,” he added.
“Having access to accurate cardiac performance data for heart patients is critical for improving outcomes,” said Samir Saba, MD, Chief of the Division of Cardiology and Co-Director, UPMC Heart and Vascular Institute at the University of Pittsburgh Medical Center. “This Cardiac Performance System (CPS) shows promise as a non-invasive alternative to right-heart catheterization. The CPS platform has the potential to revolutionize the way we diagnose and treat heart failure and pulmonary hypertension by enabling rapid, non-invasive assessment of cardiac pressures.”
Sensydia previously conducted a study at the Ronald Reagan UCLA Medical Center which contributed to the FDA clearance for its ejection fraction algorithm and another study at the OHSU Knight Cardiovascular Institute for its cardiac output algorithm.
In January 2022, Sensydia announced that CPS was granted Breakthrough Device Designation by the United States Food and Drug Administration (FDA). Sensydia plans to use data from this 225-subject study to develop the CPS pulmonary pressure algorithms.
The achievement of this 225-subject milestone comes at a time when Sensydia is closing a new round of funding. With its CPS device, Sensydia aims to democratize cardiac assessment and bring fast and safe cardiac assessment to the lowest acuity setting.