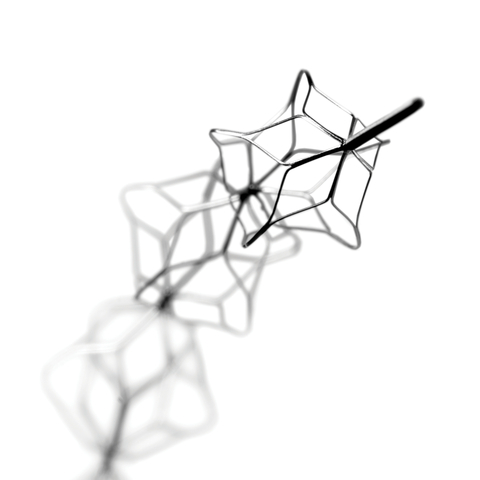
LISO VIEJO, Calif.– MicroVention, Inc., a global neurovascular company and wholly owned subsidiary of Terumo Corporation, today announced that the ERIC Retrieval Device is now commercially available in the United States for ischemic stroke treatment. ERIC received FDA 510(k) clearance on March 31, 2022. ERIC delivers thrombus control inside and outside of the device, offers no clot integration wait-time to drive procedure efficiency, and offers versatile treatment options for thrombectomy. With the introduction of the ERIC Retrieval Device, MicroVention now offers a comprehensive and streamlined portfolio of stroke solutions, delivering compatibility, versatility, and speed unlike any other.
MicroVention’s ERIC Retrieval Device is a self-expanding laser-cut clot retriever with multiple retrieval cages, located on a pusher wire delivery system. Unlike tubular stents where the ends are open, ERIC is designed to control thrombus through its cage-like design. The ERIC Retrieval Device has an atraumatic design that conforms to the natural vessel, offering soft, outward radial force, minimal vessel wall contact, and an exceptionally flexible design. ERIC is offered in three sizes that treat a wide vessel size range of 5.5 mm down to 1.5 mm and is compatible with MicroVention’s HEADWAY™ 17 and HEADWAY 21 Microcatheters. As part of the initial limited release, the first ERIC cases in the U.S. were performed by the neurovascular team at Riverside Methodist in Columbus, OH: Dr. Pema, MD,MS, FACR, Dr. Alhajeri, MD, and Dr. Budzik, MD in February 2023.
ERIC delivers safe and effective outcomes, showing similar results in successful revascularization, clinical and safety outcomes when compared to other retrieval devices.
“The ERIC Retrieval Device’s ability to rapidly interact and trap the embolus, coupled with its low-profile design, facilitates synergistic use with SOFIA catheters and reduces time to recanalization,” said Dr. Adel Malek, Chief of Neurovascular Surgery and Director of the Cerebrovascular and Endovascular Division in the department of Neurosurgery at Tufts Medical Center in Boston, MA. “We are excited to have been the first center in Massachusetts to have clinical experience using the ERIC device and since then have integrated ERIC into our stroke practice.”
Leveraging the company’s in-house R&D and manufacturing expertise and precision, MicroVention’s fully integrated and streamlined portfolio of stroke solutions delivers compatibility, versatility, and speed. In addition to the ERIC Retrieval Device announced today, MicroVention’s integrated stroke solution portfolio includes:
- SOFIA™ Catheters: Take control with renounced trackability and proven clinical performance
- BOBBY™ Balloon Guide Catheter: BOBBY adds reliable flow arrest to procedures with optimized compatibility, streamlined prep, and next-generation balloon technology
- WEDGE™ Microcatheter: Create a smooth path in extreme tortuosity for aspiration catheters with a navigation aid that minimizes the ledge effect
- HEADWAY™ Microcatheters: Engineered for excellent trackability, featuring a low-profile outer diameter, providing versatility, reliability, and fast access
- TRAXCESS™ Guidewire: Track through challenging anatomies with tip softness and flexibility
“Today’s introduction of the ERIC Retrieval Device is guided by our continued commitment to meaningful advancement in stroke innovation. ERIC underscores this commitment to offering physicians and patients vital versatility when every second counts,” said Carsten Schroeder, President and CEO, MicroVention, Inc. “We are thrilled to now offer a comprehensive, fully integrated stroke portfolio that delivers compatibility, versatility, and speed – a true standout in the neurovascular space. This is the result and honor of working side-by-side with leading physicians around the world to identify the evolving needs in patient care, and then transform those insights into innovative technologies that help to save patient lives.”