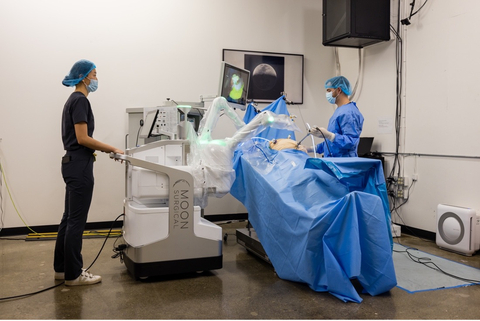
PARIS & SAN FRANCISCO– Moon Surgical, a French-American pioneer in collaborative robotics, announced today that the latest version of its Maestro System is CE Marked under the Medical Device Regulation (EU) 2017/745.
In addition to its scalability and refined aesthetics, this updated Maestro boasts several improvements over its predecessor, such as:
- Automated set-up with bedside guidance, and tailored surgeon configurations
- Cloud connectivity for a more integrated Maestro OR and ecosystem experience
- Surgeon guided hands-free scope control for optimized vision capabilities
“This latest evolution of the Maestro System focuses on manufacturing scalability, together with increased capability and has simplified both usability and training,” said Anne Osdoit, CEO of Moon Surgical, and a Partner at Sofinnova Partners. “It seamlessly integrates into OR workflows while giving surgeons more confidence and delivering an improved surgical experience.”
With its new category of robotic surgery, Moon Surgical aims not only to broaden the scale at which robotics are used but also to improve the quality of care for patients and the bottom line for healthcare providers. The Maestro System is designed to provide an accessible and enhanced version of traditional laparoscopy to over 18 million patients undergoing soft tissue procedures annually who currently lack the benefits of robotic surgical platforms.
The initial version of the Maestro System obtained its CE Mark earlier this April.