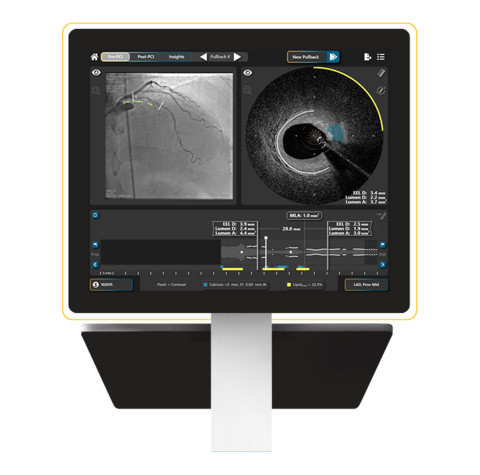
BEDFORD, Mass.– SpectraWAVE, Inc., a medical imaging company focused on improving the treatment and outcomes for patients with coronary artery disease (CAD), today announced U.S. Food and Drug Administration (FDA) 510(k) clearance for product enhancements to the HyperVue™ Imaging System. The intravascular imaging system combines next-generation DeepOCT™ images and near infrared spectroscopy (NIRS) with state-of-the-art ease of use to support physicians optimizing coronary stenting in the cardiac catheterization lab. Newly cleared product enhancements include contrast-free saline imaging, artificial intelligence algorithms to support the identification of key clinical structures of calcium and external elastic lamina (EEL), and hands-free angiographic co-registration. The latest clearance adds to the existing HyperVue toolkit, which includes artificial intelligence enabled lipid, lumen, stent, and sidebranch detection, as well as the no-flush prep Starlight™ Imaging Catheter designed for efficient setup and image acquisition in complex lesions.
“Intravascular imaging is essential to optimize patient outcomes,“ said Richard A. Shlofmitz, M.D., Chairman of Cardiology of the St. Francis Hospital and Heart Center. “I use intravascular imaging in all my coronary stenting patients, and I’m delighted to see SpectraWAVE enter the arena and push the limits of these technologies to help us better care for our patients. The combination of DeepOCT and NIRS is ground-breaking and provides important clinical information that was previously unavailable. With the recent updates that allow for contrast-free saline imaging and support image review with artificial intelligence, this product will provide significant value to both experienced physicians and those just beginning to embrace imaging.”
“HyperVue had already set a new bar for intravascular imaging, and these additions now solidify its place as a leapfrog platform in the catheterization lab,” said Eman Namati, Ph.D., Chief Executive Officer of SpectraWAVE. “Optimal stenting outcomes for patients, as demonstrated by the wealth of randomized clinical studies and meta-analyses, are predicated on the ability to acquire high-quality intravascular images and distill the data into clinical actions. We have pushed the limits of image quality with our DeepOCT-NIRS technology, created a simple low-profile no-flush prep catheter, and enabled long and fast pullbacks. We have now expanded our artificial intelligence algorithms to support EEL-based vessel measurements, fully automated angiographic co-registration to map findings back to the angiogram, and enable saline imaging to mitigate the need for additional contrast use. The team is thrilled about these new additions and their ability to have a meaningful and broad impact on patient care. This next generation intravascular imaging product serves as the backbone of the HyperVue platform as a diagnostic hub for interventionalists, with the addition of a Fractional Flow Reserve physiology assessment on its way.”
Intravascular imaging has become an essential tool to optimize the approximately 1 million coronary stenting procedures per year in the United States, providing key insights into plaque morphology, plaque modification decisions, stent and balloon sizing and landing zone selection, confirmation of treatment optimization, and future adverse event risk. Clinical evidence for the use of intravascular imaging continues to grow, culminating with a recent meta-analysis presented at the European Society of Cardiology 2023 Congress that incorporated 12,428 patients from 20 randomized trials of intravascular imaging guided stenting compared with angiography alone. Intravascular imaging guidance of stenting was associated with a significant improvement in patient outcomes, including reductions of target lesion failure, cardiac death, target vessel myocardial infarction, and stent thrombosis by 31%, 46%, 20%, and 52%, respectively.