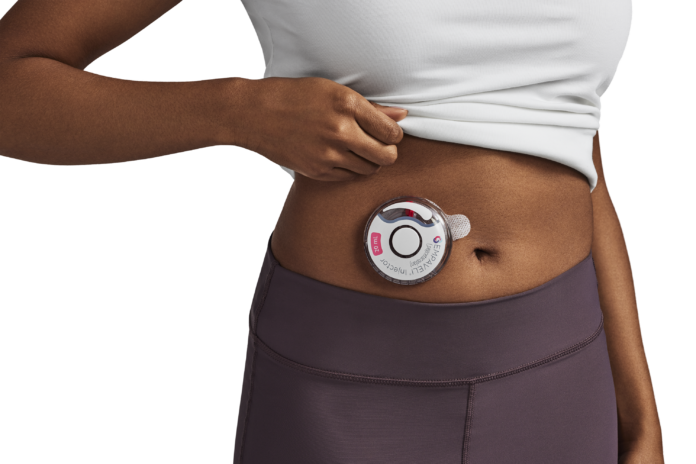
WALTHAM, Mass.– Apellis Pharmaceuticals, Inc. (Nasdaq: APLS) today announced that the U.S. Food and Drug Administration (FDA) has approved the EMPAVELI® Injector. The EMPAVELI Injector is a compact, single-use, on-body device designed to enhance self-administration of EMPAVELI (pegcetacoplan), which is approved for adults with paroxysmal nocturnal hemoglobinuria (PNH).
“EMPAVELI continues to demonstrate its potential to elevate the standard of care, including rapid and sustained improvements of PNH disease measures. Now, we are further enhancing the patient experience with the approval of the EMPAVELI Injector, an innovative and first-of-its-kind, high-volume injector,” said Peter Hillmen, M.B. Ch.B., Ph.D., head, rare disease advisor, Apellis. “This approval is an example of how we are continually innovating with patients at the forefront.”
The EMPAVELI Injector is the first high-volume (20mL), subcutaneous on-body delivery system which features several advances to streamline self-administration. The compact device offers patients greater mobility when administering EMPAVELI. A push button starts the injection, and the hidden needle automatically retracts upon dose completion. The EMPAVELI Injector is developed in collaboration with Enable Injections, based on the enFuse® Syringe Transfer System.
“The approval of the EMPAVELI Injector is an exciting advancement. People living with PNH can now receive the benefits of EMPAVELI via on-body treatment administration, allowing for greater mobility,” said Carlos de Castro, M.D., professor of medicine, Duke University. “With the EMPAVELI Injector, patients can seamlessly integrate EMPAVELI treatment into their daily lives, whether that is at home or on the go.”