BOSTON– Desktop Health — the trusted production-grade medical 3D printing brand of Desktop Metal, Inc. (NYSE: DM) — today announced an even stronger formulation of its Flexcera resin for 3D printing gingiva, Flexcera Base Ultra+, which is used to fabricate full and partial removable dentures.
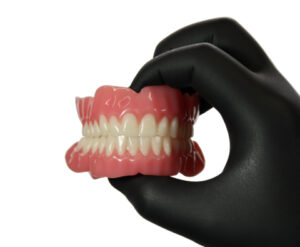 New Flexcera Base Ultra+ is 50% stronger than its predecessor2 with 70% greater resistance to deformation vs. ISO standards3. This improved flexural strength allows for the creation of thinner wall and socket designs, enhancing resistance to deformation for long-term durability, fit, and overall patient comfort. This powerhouse formulation better aligns with design expectations in the industry and also resists staining for confidence in long-term aesthetics.
New Flexcera Base Ultra+ is 50% stronger than its predecessor2 with 70% greater resistance to deformation vs. ISO standards3. This improved flexural strength allows for the creation of thinner wall and socket designs, enhancing resistance to deformation for long-term durability, fit, and overall patient comfort. This powerhouse formulation better aligns with design expectations in the industry and also resists staining for confidence in long-term aesthetics.
“We are witnessing exponential growth in the adoption of these restorative solutions,” said Lou Azzara, President of Desktop Health. “When paired with the Einstein and the Einstein Pro XL printing systems, which exhibit best-in-class speed and accuracy, Flexcera Base Ultra+ prints 26% faster3 than its predecessor to scale production without compromising quality.”
In testing, Einstein Pro XL produces 21 Flexcera Base Ultra+ denture arches in about 90 minutes3 and reduces post-processing time for a highly efficient workflow.
“New Flexcera Base Ultra+ demonstrates how we listen to customers and give them what they’re asking for, such as a firmer final product in an industry favorite shade,” said Ric Fulop, Founder and CEO of Desktop Metal. “With more dental professionals than ever adopting a digital workflow and an aging population, the popular Flexcera family fits a growing need in the marketplace.”
Sources:
- Maximize Market Research, Forecast on the Global Dentures Market (2024-2030)
- Internal throughput testing, case and depth dependent, results on file 12/2023.
- Resistance to deformation defined by Flexcera Base Ultra+ DIN EN ISO 20795-1 flexural strength (MPa) when used in the Otoflash curing unit, results on file, 08/2023.


