SILVER SPRING, Md. & RESEARCH TRIANGLE PARK, N.C.– United Therapeutics Corporation (Nasdaq: UTHR), a public benefit corporation, today announced the world’s first successful transplant of a UThymoKidney™, which the company produced, into a living person on April 12, 2024. This transplant represents several historic firsts for transplantation:
– The first-ever transplant of a xenothymokidney into a living human recipient;
– The first-ever combined mechanical heart pump and organ transplant; and
– The first-ever xenotransplant into a living human using only FDA-approved immunosuppressive medicines
The transplant is the third xenotransplant using United Therapeutics’ xeno organs, following two successful UHeart™ transplants at the University of Maryland Medicine in 2022 and 2023.
The transplant was authorized by U.S. Food and Drug Administration (FDA) under the expanded access pathway and performed by surgeons at NYU Langone Health led by Robert Montgomery, M.D., DPhil. The patient, a 54-year-old woman from New Jersey, suffers from heart and kidney failure. The combination of several chronic medical conditions, coupled with a lack of available human organs for transplant, prevented her from qualifying for human heart and kidney transplants.
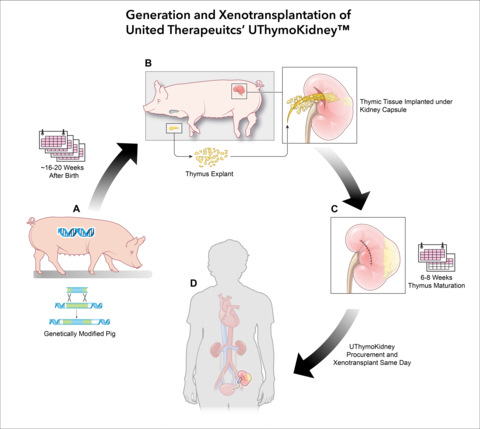
United Therapeutics’ xenothymokidney, known by the proposed trade name UThymoKidney, is an investigational-stage xenokidney from a pig with a single genetic edit, together with tissue from the same pig’s thymus. The use of the pig’s thymus tissue is intended to condition the recipient human’s immune system to recognize the UThymoKidney as “self” and reduce the likelihood of rejection.
The single genetic modification in the pig is the inactivation, or “knock-out”, of the gene responsible for the synthesis of alpha-gal, a sugar on the surface of cells that can cause the immediate rejection of an organ when transplanted into the human body. Because tissues from pigs containing this modification do not contain detectable levels of alpha-gal, United Therapeutics refers to materials derived from this pig as GalSafe®.
The GalSafe pig was developed by Revivicor, Inc., a subsidiary of United Therapeutics. In December 2020, this pig line was approved by the FDA for use as human food or as a potential source for biomedical purposes, with this being the first investigational biomedical use in a living human.
“This historic transplant builds on the base of knowledge that the teams at United Therapeutics and our academic collaborators have established over the past two decades and demonstrates the potential utility for xeno organs to revolutionize the way patients with end-stage organ disease are managed in the future,” said Leigh Peterson, Ph.D., Executive Vice President, Product Development & Xenotransplantation at United Therapeutics. “We look forward to continuing our dialogue with the FDA with the goal of starting human clinical studies for xenotransplantation in 2025.”
According to the U.S. Health Resources and Services Administration, around 110,000 Americans are currently waiting for an organ transplant, and more than 6,000 patients – 17 every day – die each year before receiving one. More than 89,000 patients are waiting for kidneys, close to 10,000 for livers, over 3,400 for hearts, and almost 1,000 for lungs, with many more patients suffering from end-stage organ failure who are ineligible for the strict organ transplant waiting list who could benefit from a readily available supply of organs on demand.
“I am pleased and impressed that decades of research into expanding the supply of kidneys have resulted in this historic, successful xenokidney transplant using United Therapeutics’ gene editing and thymokidney technology,” said Dr. Louis Sullivan, Secretary of the United States Department of Health and Human Services in President George H.W. Bush’s administration, member of the United Therapeutics Board of Directors, and Chair of its Scientific Advisory Board.
“I am so proud of the many scientists and surgeons working with United Therapeutics on its xenotransplantation programs,” said Gov. Tommy Thompson, Secretary of the United States Department of Health and Human Services in President George W. Bush’s administration and member of United Therapeutics’ Board of Directors. “This major breakthrough is a revolutionary step forward in our quest to create an unlimited supply of transplantable organs.”
United Therapeutics’ organ manufacturing efforts consist of four platforms – xenotransplantation, regenerative medicine, 3D organ bioprinting, and bio-artificial organs – encompassing four different organs – hearts, kidneys, livers, and lungs. These groundbreaking programs are intended to address the ongoing shortage of transplantable organs for patients with end stage organ disease.
United Therapeutics initiated its xenotransplantation research work in 2011 and currently employs close to 50 scientists and support staff advancing xenotransplant science with three different organ programs: the UHeart xenoheart, the UThymoKidney, a one-gene modified kidney and thymus, and the UKidney™, a 10-gene modified kidney. In 2024, the company inaugurated the world’s first clinical-scale designated pathogen-free facility in Christiansburg, Virginia to support future clinical xenotransplantation studies with a capacity of approximately 125 organs per year.
To date, 11 xenotransplantation procedures using United Therapeutics’ UHearts, UThymoKidneys, and UKidneys have been performed in living and brain-dead human recipients: two living human recipients of UHearts, one living recipient of a UThymoKidney, six brain-dead UKidney and UThymoKidney recipients, and two brain-dead UHeart recipients. United Therapeutics has built on its history of innovation in xenotransplantation with strong research collaborations with top academic medical centers including NYU Langone Health, the University of Maryland Medicine, Johns Hopkins Medicine, and the University of Alabama at Birmingham.
United Therapeutics is preparing for clinical trials of its xenokidney, xenothymokidney, and xenoheart products, following completion of ongoing preclinical studies required by the FDA.

