LUXEMBOURG & AACHEN, Germany– The European Investment Bank (EIB) is providing €20 million in venture-debt financing to German medical-technology company Protembis to develop a next-generation device for protecting the brains of patients who undergo certain heart treatments.
The funding is to support clinical trials, research, development and market access for the “ProtEmbo” cerebral embolic protection system. ProtEmbo is a filter device that deflects embolic material away from arteries to the brain during left-sided heart procedures including transcatheter aortic valve replacement (TAVR), countering risks including stroke and cognitive decline.
“This agreement demonstrates our commitment to supporting companies such as Protembis that aim to improve the health and well-being of European citizens. Their innovative technology, developed in Europe, will save patients who have to undergo heart surgery, from suffering grave side effects like cerebral embolic events”, said EIB Vice-President Nicola Beer.
The EIB accord with Protembis is supported by the InvestEU programme to trigger more than €372 billion in additional investment in new technologies until 2027. The deal is aligned with the InvestEU objective of promoting research, development and innovation.
“We are pleased to announce the signature of this agreement with the EIB,” said Protembis Co-Chief Executive Officers Karl von Mangoldt and Conrad Rasmus. “We would like to recognise the hard work, skill and professionalism of the EIB team while thanking our existing investors and our board for their unwavering belief in the benefits of this additional financing facility.”
TAVR is an x-ray guided procedure to replace an aortic valve that has narrowed and doesn’t open fully. TAVR is minimally invasive, meaning it uses smaller incisions than open-heart valve surgery. Globally, around 430,000 patients with severe aortic stenosis are expected to be treated with TAVR by 2025.
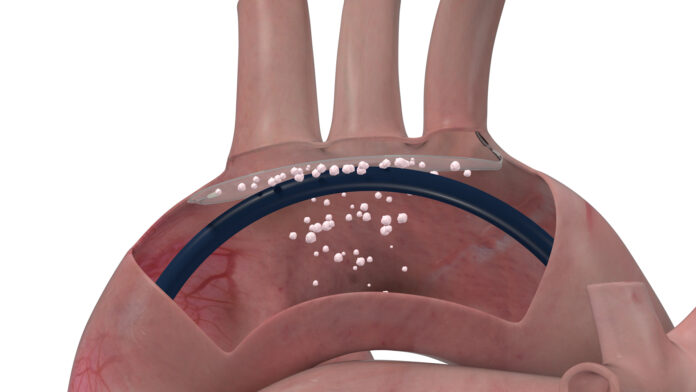
A significant complication of TAVR is the risk of dislodging debris that lines the aortic arch and the old aortic valve. This debris can travel to the brain via three major arteries that originate in the roof of the aortic arch. It can block the smaller vessels in the brain and create lesions, which may cause stroke or contribute to acceleration of cognitive decline.
ProtEmbo is inserted during TAVR via the artery in the left wrist, and lines the roof of the aortic arch, shielding the brain from the dislodged debris.
In March 2024, Protembis completed a €30 million Series B financing round to advance a pivotal FDA approved Investigational Device Exemption study called the ProtEmbo trial. The trial will enrol between 250 and 500 patients undergoing TAVR in Europe and the US. The study aims to show the superiority of ProtEmbo by randomising against a hybrid control group: half of this control-group receiving no protection and half receiving the current predicate system called “Sentinel”.