FRANKLIN, Mass.– Heidelberg Engineering, a global leader in ophthalmic imaging and healthcare data solutions, announces FDA clearance of SPECTRALIS® OCTA Module with SHIFT technology, which reduces acquisition time by 50%1. The preset OCTA speed of 125 kHz is designed to help streamline workflow, enhance clinical efficiencies, and maintain Heidelberg image quality.
“While SPECTRALIS OCTA Module has always been a safe and cost-effective precursor to traditional angiography, delivering unparalleled resolution, many eye care practitioners have hesitated to fully adopt this advanced technology into daily clinical practice because of the acquisition time,” says Ram Liebenthal, General Manager USA at Heidelberg Engineering. “We are thrilled to introduce the next generation of OCTA that increases the acquisition speed without sacrificing image quality, thus making it a more versatile clinical tool in the diagnoses and management of retinal diseases.”
Combined with a more powerful OCT engine, updated graphics processing technology, and software optimization, SHIFT technology delivers increased speed, maintains data integrity, and improves performance. The technology is available exclusively on third generation SPECTRALIS devices.
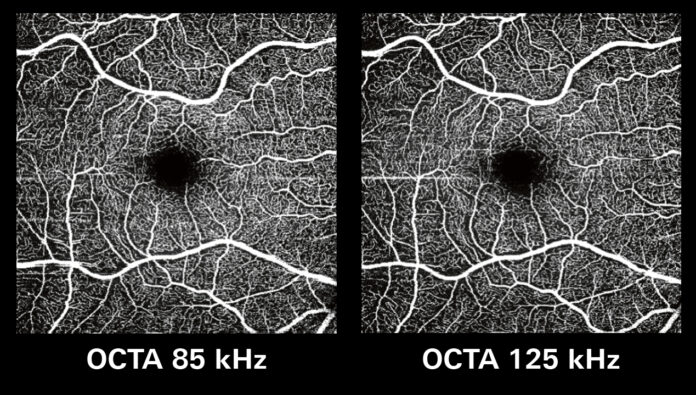
Both the 85 kHz preset for structural OCT and the 125 kHz preset for OCT angiography (OCTA) allow eye care practitioners to complete their patient exams more efficiently. Furthermore, the 125 kHz OCTA image acquisition rate allows for visualization of flow, even in miniscule vessels, while minimizing artifacts, resulting in sharp and detailed images of the capillary network.
Dennis M. Marcus, MD, principal investigator of the clinical trial, said, “Reducing the scanning time for OCTA without diminishing the resolution is a huge step forward, not only for SPECTRALIS but for the industry. Speed plus image quality eliminates the barriers and opens the door for mainstream adoption. I think we will start to see more doctors warm to the idea of trying OCTA as part of the routine clinical work-up for at-risk patients.”