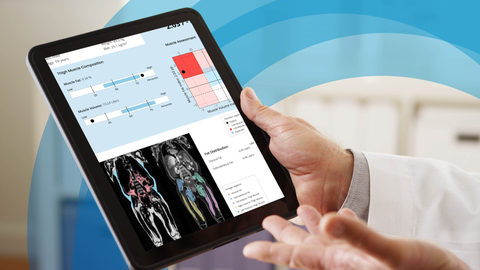
LINKÖPING, Sweden– A recent publication in the Annals of Hepatology utilizing the AMRA® MAsS Scan, a body-composition analysis service powered by the FDA 510(k) cleared AMRA® Profiler 4 device, explored the feasibility of using AMRA’s specialized MRI-based analysis for generating muscle composition assessments in patients with end-stage liver disease (ESLD). These findings come during a critical period in liver disease research, as methods to combat challenges associated with identifying and treating sarcopenia and frailty in ESLD have become front-of-mind for researchers and clinicians, in an effort to address unmet needs in the highly-burdened liver disease population.
AMRA® MAsS Scan is a unique, specialized rapid neck-to-knee MRI protocol and automated image analysis technique that provides volumetric assessments of skeletal muscles and muscle fat infiltration. The study investigators at Northwestern University and the University of Pittsburgh enrolled 18 liver transplant candidates with ESLD, who then underwent muscle composition assessment utilizing the AMRA® MAsS Scan. The investigators also tested if the muscle assessments correlated with commonly used measures of frailty and sarcopenia in this patient group.
Professor Andres Duarte-Rojo, MD, Director of Liver Transplantation and Living Liver Donor for the Comprehensive Transplant Center at Northwestern Medicine, stated: “Apart from being easy to interpret and of great anatomic value, the MAsS Scan results correlated with well-validated parameters of physical deconditioning and visceral adiposity. The study showed that for skeletal muscle wellness, both quantity and quality are relevant and can further expand the phenotype of chronic liver disease. It is thus possible to consider that an adverse muscle composition by MAsS Scan can be used not only for anatomic sarcopenia but as a surrogate for muscle functionality and metabolic health, a concept that will need to be confirmed in larger studies.”
The researchers report that the average total time spent by the patients in the MRI scanner was just under 15 minutes. In addition, no adverse events were reported during the MRI scanning, suggesting that the MRI-data used in AMRA® MAsS Scan are safely obtainable and can be analyzed by AMRA® Profiler 4, even in the presence of severely decompensated ESLD cases. 41% of the patients were reported to have the adverse muscle composition (AMC) phenotype, characterized as having high muscle fat infiltration and low muscle volume. The AMC group was more likely to have undergone a large volume paracentesis, and performed significantly worse on functional tests such as the 6-minute walk test compared to their non-AMC counterparts, demonstrating a correlation between AMC and reduced function in ESLD patients.
While the pilot study is the first to assess the feasibility of using AMRA® Profiler 4 in patients with ESLD, AMRA is continuing to pioneer the use of MRI-based body composition biomarkers that when interpreted by a physician, may assist in diagnosis. AMRA® Profiler 4’s recent EU MDR certification brings this assistance via powerful and unique muscle composition biomarkers one step closer to patients everywhere.