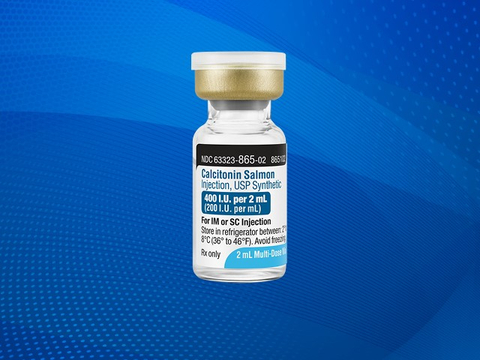
LAKE ZURICH, Ill.– Fresenius Kabi, an operating company of Fresenius, announced today it has introduced Calcitonin Salmon Injection, USP Synthetic, a calcium regulator, for the treatment of symptomatic Paget’s disease of the bone and hypercalcemia. The drug is also used to treat postmenopausal osteoporosis when alternative treatments are not suitable. Fresenius Kabi Calcitonin Salmon Injection is formulated, filled and packaged in the United States.
Available in 400 I.U./2 mL (200 I.U./mL) vials, the new product adds to the company’s generic injectables portfolio that delivers affordable treatments and a reliable supply for patient care.
“We continue to broaden our portfolio of essential medicines in the U.S. with the launch of Calcitonin Salmon. At Fresenius Kabi, every product addition further underscores our commitment to the ‘supply chain of care’ for patients,” said Arunesh Verma, president of Fresenius Kabi Region U.S. and member of the executive leadership team at Fresenius Kabi AG.
Since 2017, Fresenius Kabi has invested nearly $1 billion to modernize and expand its advanced U.S. pharmaceutical production and distribution facilities to strengthen supply chains of care in and for the U.S.