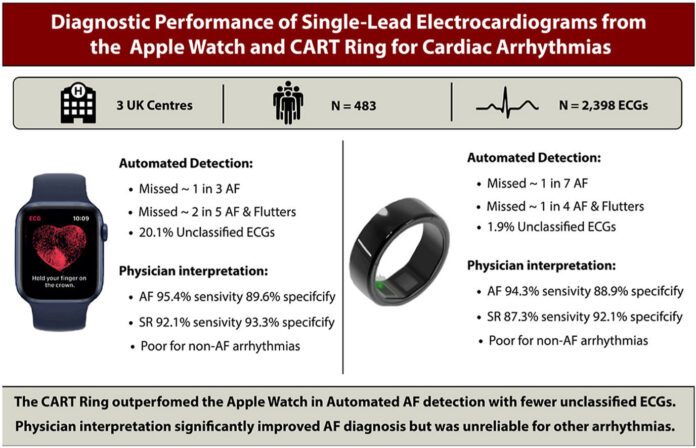
SEOUL, South Korea– South Korean health-tech company Sky Labs has announced that its smart ring device, CART-I, outperformed the Apple Watch in detecting atrial fibrillation (AFib) in a UK-based clinical study. The results, published in the international peer-reviewed journal Heart Rhythm O2, found that CART-I achieved an 84.6% sensitivity rate compared to the Apple Watch’s 69.1%.
The comparative study was conducted across three major UK hospitals—Oxford University Hospital, University Hospital Southampton, and Queen Elizabeth Hospital—and involved approximately 500 patients. Both devices were evaluated using the same clinical protocols, including the analysis of single-lead electrocardiogram (SL-ECG) data interpreted by physicians.
While CART-I demonstrated superior automatic detection of AFib, both devices showed high accuracy when SL-ECG readings were interpreted by physicians. The Apple Watch recorded a physician-interpreted sensitivity of 95.4%, while CART-I closely followed at 94.3%.
“This study offers strong external validation of CART-I’s performance in detecting atrial fibrillation,” said Jack Lee, CEO of Sky Labs. “Combined with our CART BP device for hypertension, these results reinforce our commitment to advancing wearable cardiovascular monitoring technologies.”
Unlike previous studies that primarily focused on AFib, this research also included other arrhythmias such as atrial flutter and atrial tachycardia. However, researchers emphasized that wearable devices are not yet adequate for diagnosing complex arrhythmias independently, and physician oversight remains crucial.
In a separate achievement, Sky Labs recently received South Korea’s prestigious IR52 Jang Young-sil Prime Minister’s Award for its cuffless blood pressure monitoring device, CART BP. The award recognized the device for its economic value, technological innovation, originality, and independence.
CART BP enables continuous, finger-based blood pressure monitoring, eliminating the need for traditional arm cuffs. Approved by South Korea’s Ministry of Food and Drug Safety in 2023, it also received coverage under the national health insurance system in 2024. As of April 2025, the device is in use at more than 1,300 hospitals and clinics across the country.
Sky Labs says it plans to continue expanding its suite of non-invasive cardiovascular monitoring tools, positioning itself as a key innovator in the global health-tech space.