Montpellier, France– French medtech company Womed has announced the European launch of its breakthrough device, Womed Leaf®, designed to prevent intrauterine adhesions (IUA), a major cause of infertility. The rollout follows licensing agreements with Kebomed Europe and Saesco Medical, enabling distribution across 14 countries.
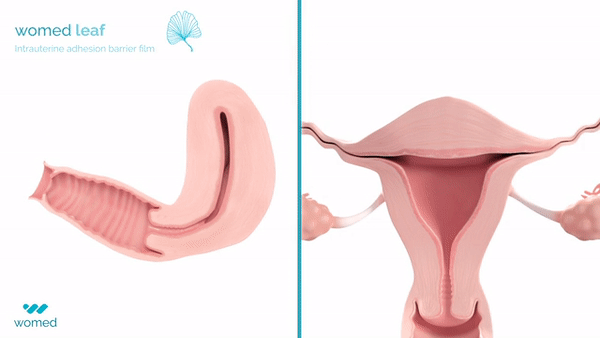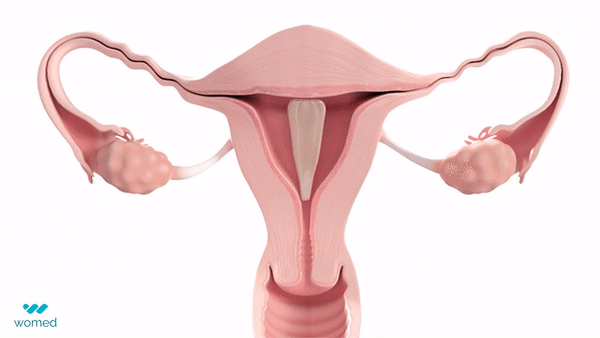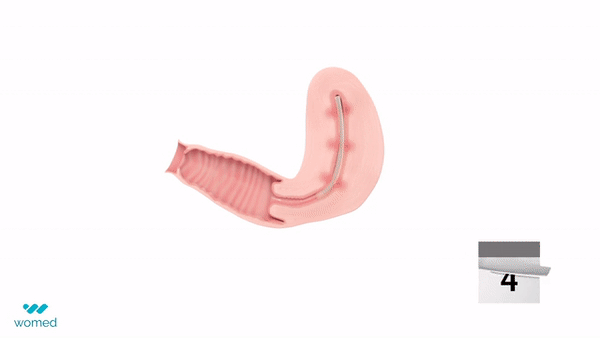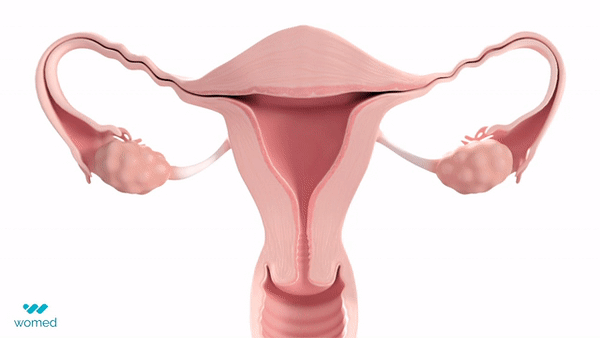
Womed Leaf® is the first intrauterine adhesion barrier film on the market. It aims to protect women’s fertility by preventing scar tissue from forming inside the uterus after surgical procedures such as dilation and curettage or fibroid removal. IUAs, which occur in up to 45% of such procedures, are often linked to infertility, miscarriages, and chronic pelvic pain.
The device is a soft, flexible film made from Womed’s proprietary polymer. It is inserted into the uterus like an IUD following a procedure and expands to prevent the uterine walls from adhering. After one week, the film naturally dissolves and is painlessly expelled from the body.
Womed Leaf® has shown promising results in clinical studies, including the PREG2 trial, which enrolled 160 patients with moderate to severe IUA. The trial confirmed the device’s effectiveness in significantly reducing adhesion recurrence rates.
“These partnerships mark the first step of a global launch that will make Womed Leaf the new gold standard in intrauterine adhesion prevention and treatment,” said Gonzague Issenmann, Womed Co-founder and CEO. “Kebomed Europe and Saesco Medical’s strong presence in women’s health will be instrumental in making this innovation widely available.”
Kebomed Europe will manage distribution in France, Germany, Sweden, Denmark, Norway, Finland, Austria, and Switzerland, while Saesco Medical will cover Spain, Italy, Portugal, Belgium, the Netherlands, and Luxembourg.
Lars Melbye, Managing Director of Kebomed Europe, highlighted the device’s potential: “Womed Leaf addresses a significant unmet need in women’s health, and we are excited to bring it to our customers.” Saesco Medical CEO Jordi Fluvia added, “This is true innovation in a field that has seen little progress in decades.”
The deal was facilitated by Staatz Business Development & Strategy, acting as Womed’s business development partner.
With the European launch underway, Womed aims to position its device as a new standard of care for women recovering from uterine surgeries, offering new hope for fertility preservation and reproductive health.