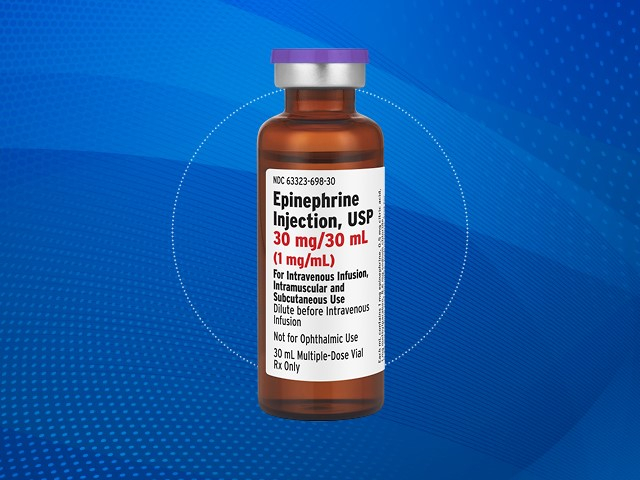
LAKE ZURICH, Ill.– Fresenius Kabi has expanded its line of emergency care medications with the U.S. launch of Epinephrine Injection, USP, in a 30 mg per 30 mL multi-dose vial. The new product adds to the company’s growing portfolio of epinephrine injectables and aims to support hospitals and healthcare providers with cost-effective, domestically manufactured treatment options.
The newly introduced multi-dose vial is intended for the emergency treatment of Type 1 allergic reactions, including anaphylaxis, in both adults and pediatric patients. It is also approved to help increase mean arterial blood pressure in adult patients experiencing hypotension associated with septic shock.
This launch follows the company’s December 2024 introduction of the first generic version of Epinephrine, USP, in a 1 mg per mL vial. Together, these products reflect Fresenius Kabi’s commitment to broadening access to essential emergency medications.
“We are pleased to offer our customers a cost-effective solution that supports quality patient care,” said Arun Verma, President of Fresenius Kabi Region U.S. and member of the company’s executive leadership team. “At Fresenius Kabi, we are continually striving for new solutions as a trusted partner to the healthcare community.”
Manufactured in the United States, the new epinephrine product underscores Fresenius Kabi’s dedication to domestic pharmaceutical production. Since 2017, the company has invested nearly $1 billion to expand and modernize its U.S. manufacturing and distribution infrastructure. Today, more than 70% of the drug units Fresenius Kabi ships in the U.S. are listed on the FDA’s Essential Medicines List.
The release of the 30 mg per 30 mL vial offers greater flexibility in dosing and administration for healthcare providers managing emergencies where rapid, reliable epinephrine delivery is critical.