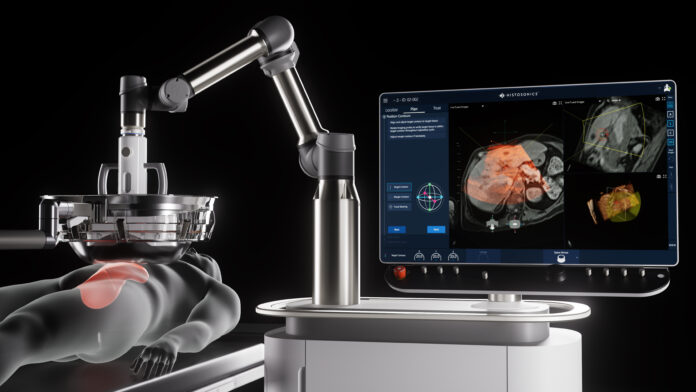
MINNEAPOLIS — HistoSonics has announced that its non-invasive Edison Histotripsy System has received early market access in Great Britain under the Unmet Clinical Need Authorisation (UCNA), part of the UK’s Innovative Devices Access Pathway (IDAP) program. The decision allows limited and controlled use of the device for patients with liver tumors, offering new hope to individuals with few effective treatment options.
The Edison System, which uses a non-thermal, focused ultrasound technique to mechanically destroy tumors without the need for surgery, radiation, or systemic therapies, is the first and only medical platform to harness histotripsy technology. It allows physicians to plan and monitor treatment in real time, offering precise tumor targeting with minimal invasiveness.
The UK’s Medicines and Healthcare products Regulatory Agency (MHRA) granted the special access following a rigorous evaluation under the IDAP pilot, which was launched to fast-track the adoption of innovative medical devices into the National Health Service. HistoSonics’ Edison System was one of only eight technologies selected for the 2024 cohort based on its potential to address significant public health needs.
“This is a tremendous milestone for HistoSonics and reflects growing recognition of histotripsy’s potential to transform care for patients with liver tumors,” said Mike Blue, President and CEO of HistoSonics. “We are proud to collaborate with the NHS and UK regulators to provide access to patients who may otherwise have limited treatment choices.”
The UK approval follows the system’s U.S. FDA De Novo clearance in October 2023. Since then, the Edison System has been adopted by leading academic hospitals and health systems in the United States, and HistoSonics is currently running clinical trials aimed at expanding the technology’s use to other cancers, including kidney and pancreatic tumors.
In the UK, HistoSonics has already developed strong clinical partnerships through early trials involving liver and kidney cancers, laying the groundwork for broader adoption. As part of the UCNA pathway, the company will work with MHRA to monitor patient outcomes and collect real-world safety and performance data as part of a post-market surveillance effort.
HistoSonics is also pursuing European CE marking to facilitate wider commercial access across the UK and continental Europe. The company believes that the Edison System’s unique capabilities and growing clinical evidence position it as a breakthrough option for tumor treatment, especially for patients who are ineligible for traditional therapies.