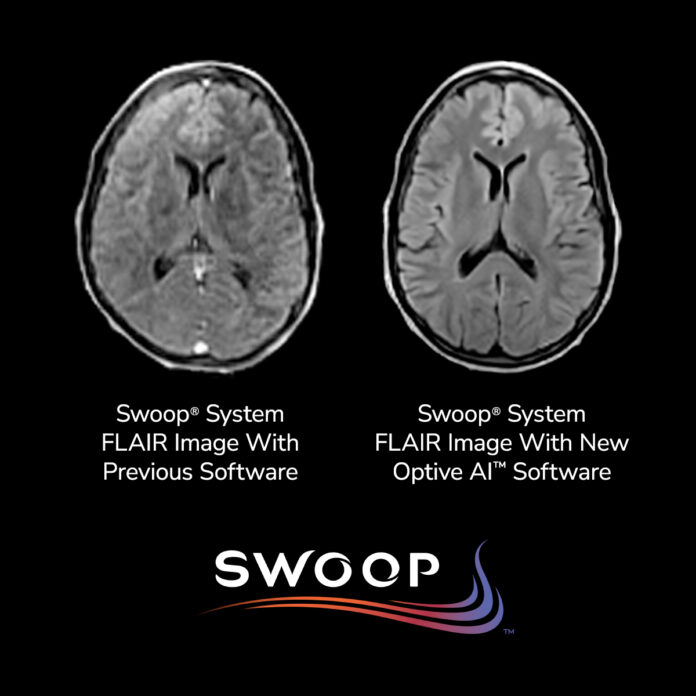
GUILFORD, Conn. — Hyperfine, Inc., the company behind the world’s first FDA-cleared AI-powered portable brain MRI system, has announced U.S. Food and Drug Administration (FDA) clearance for its latest software platform, Optive AI™, which delivers the most significant image quality improvement to date for its Swoop® system.
The new software, now in its tenth-generation release, represents a major technological advancement in ultra-low-field MR imaging. Optive AI™ enhances every stage of the imaging process—including noise reduction, image acquisition, reconstruction, and post-processing—resulting in clearer, more uniform brain scans with sharper anatomical detail.
“This software release marks the beginning of a transformative era for Hyperfine,” said Rafael O’Halloran, Vice President of Technology. “The advanced AI algorithms dramatically elevate image quality at ultra-low field strength, enabling more confident diagnoses at the point of care.”
Initial deployments of the software at select clinical sites earlier this year generated highly positive feedback, with clinicians noting image clarity that approaches the quality of traditional 1.5T MRI systems. The improved resolution is expected to enhance diagnostic confidence in a variety of care settings.
Optive AI™ was previewed at the American Society of Neuroradiology annual meeting, where it was met with strong support from the clinical community. The FDA clearance is seen as a pivotal milestone not just for Hyperfine’s product roadmap, but also for the broader adoption of portable MRI systems in both hospital and outpatient settings.
“This is a critical inflection point in the evolution of Swoop® system technology,” said Tom Teisseyre, Hyperfine Chief Operating Officer. “Optive AI™ is the first of two major technology milestones we’ve targeted for this year. These enhancements will be central to our 2025 growth strategy, including expansion into new hospital departments and entry into neurology practices.”
Hyperfine plans to begin rolling out the Optive AI™ software to existing accounts in the third quarter of 2025, marking a new chapter in point-of-care brain imaging technology.