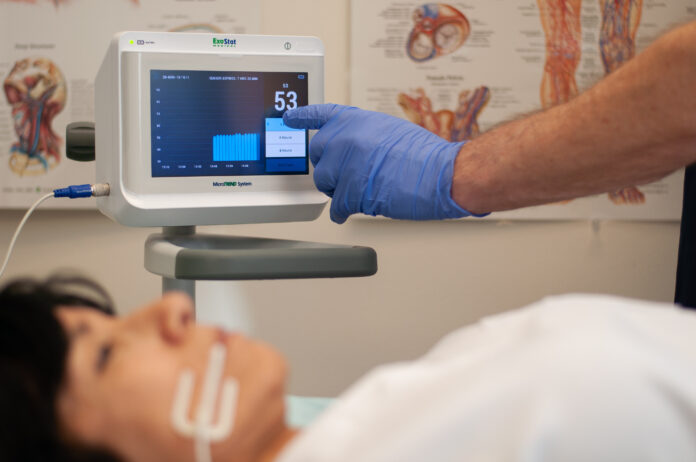
PRIOR LAKE, Minn.– ExoStat Medical, Inc. announced the successful completion of the world’s first observational study evaluating its MicroTREND System in the intensive care setting. The study supports the use of oral mucosal partial pressure of CO₂ (PomCO₂) as an early indicator of septic shock in critically ill patients—a development that could reshape how microcirculatory dysfunction is monitored in real time.
Conducted at the Second Affiliated Hospital of Anhui Medical University in Hefei, China, the IRB-approved study enrolled 23 patients with septic shock. It assessed the clinical utility of PomCO₂ monitoring using ExoStat’s MicroTREND System, comparing it with established indicators such as serum lactate levels, the current gold standard for evaluating tissue perfusion.
“This first-ever study marks a major milestone for critical care, for ExoStat, and for me personally,” said Dr. Wanchun Tang, Senior Technology and Medical Advisor for ExoStat. “For the first time, physicians were able to observe the very early signs of tissue hypoperfusion in real time at the bedside. This could fundamentally improve how we respond to septic shock—where every second counts.”
ExoStat’s MicroTREND device is a patented, FDA-cleared system that uses a non-invasive microsensor placed on the buccal tissue inside the cheek to continuously measure PomCO₂. The approach avoids the need for needles, blood samples, or catheter-based monitoring, offering clinicians immediate insight into microcirculatory function without delay.
According to Dr. Tang, traditional macrohemodynamic monitoring tools often miss the early stages of circulatory failure at the tissue level. “This study confirms what decades of research have suggested: we can now detect microcirculatory dysfunction well before systemic collapse,” he said. “It’s a breakthrough moment that transforms hypothesis into observable, actionable data.”
The observational study’s findings are currently being prepared for publication, and ExoStat is now preparing for the second phase of pre-market clinical testing. The company plans to partner with major research institutions to further explore the impact of existing care standards on microcirculation and to refine the role of PomCO₂ monitoring in clinical protocols.
“This study achieved the ultimate objective of ExoStat’s mission—to give frontline clinicians real-time visibility into the microcirculatory status of their most critically ill patients,” said Jim Hays, CEO of ExoStat. “We’re now entering a new phase of testing and collaboration to deepen our understanding and continue advancing care for patients in shock.”
The results suggest that PomCO₂, as measured by MicroTREND, may serve as a powerful adjunct to existing diagnostic tools, offering earlier warnings of deteriorating tissue perfusion in septic patients—an innovation that could ultimately save lives.