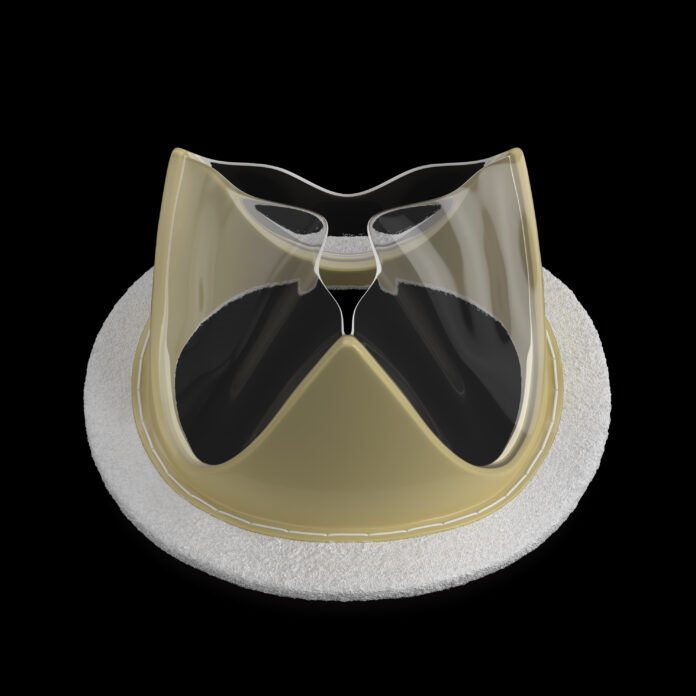
SALT LAKE CITY — Foldax Inc. has received regulatory approval in India for its TRIA™ Mitral Valve, marking a historic milestone as the first commercial polymer heart valve available to patients anywhere in the world. The approval was granted by India’s Central Drugs Standard Control Organization (CDSCO), with local manufacturing to be carried out by Dolphin Life Science India LLP.
Unlike traditional heart valves made from animal tissue or metal, which can present long-term risks like calcification or the need for lifelong blood thinners, the TRIA Mitral Valve is made from Foldax’s proprietary LifePolymer™. Engineered at the molecular level, LifePolymer is designed to be calcium-resistant, biostable, and biocompatible, offering enhanced durability without the need for chronic anticoagulation therapy.
“Approval of the TRIA Mitral Valve represents a pivotal shift in heart valve therapy,” said Foldax Executive Chairman and CEO Ken Charhut. “This innovation not only introduces a new class of materials into cardiac surgery but also provides a much-needed alternative for patients across the globe—particularly in regions with limited access to repeat surgeries.”
The TRIA Mitral Valve is robotically manufactured, a process that ensures precision and scalability, and is aimed at overcoming the performance limitations of traditional mechanical and tissue valves. Clinical research conducted in India has shown the valve delivers stable blood flow dynamics, improved safety, and enhanced patient quality of life.
Dr. Kaushal Pandey, principal investigator of the Indian clinical trial and a cardiac surgeon at P.D. Hinduja Hospital in Mumbai, praised the valve’s potential to serve a wide patient population, including younger individuals and women of childbearing age—groups particularly affected by mitral valve disease due to rheumatic fever.
Foldax plans to expand its LifePolymer platform and robotic manufacturing approach into transcatheter and other surgical valve applications, signaling its broader ambition to disrupt the conventional heart valve market and address unmet clinical needs globally.