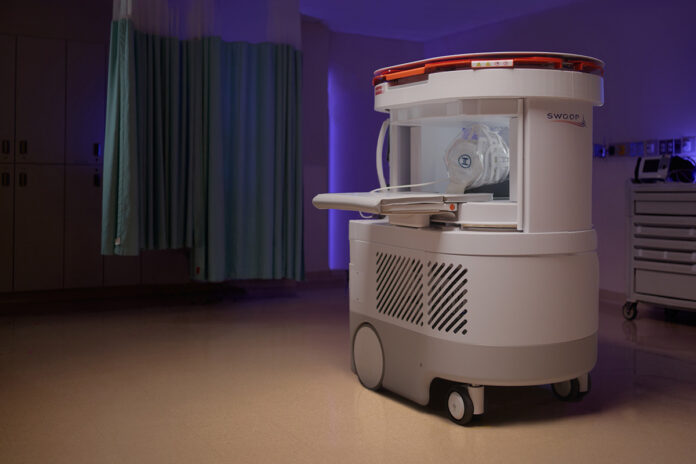
Guilford, Conn. — Hyperfine, Inc. has received FDA clearance for its next-generation Swoop® Portable MRI system, marking a major technological milestone in accessible brain imaging. Powered by the company’s newly developed Optive AI™ software, the redesigned system delivers significantly improved image quality, speed, and usability—aiming to redefine how and where MRI scans can be performed.
The new Swoop® system reflects five years of global, real-world experience and incorporates cutting-edge enhancements tailored for clinical efficiency and patient comfort. With the highest signal-to-noise ratio of any previous Swoop model and integrated AI algorithms, the updated system delivers sharper image resolution, greater uniformity, and faster scan acquisition times. These advances are designed to expand adoption of portable MRI across diverse healthcare environments—from major hospitals to neurology offices and even rural care settings.
Hyperfine President and CEO Maria Sainz described the clearance as a defining moment for the company. “This is the most significant innovation in our history,” she said. “The performance of the new Swoop® system, combined with Optive AI™, positions us to accelerate adoption across the U.S. in both hospital and outpatient settings. We believe patients and providers alike will benefit from its improved image quality, ease of use, and broader clinical accessibility.”
Designed with a patient- and user-centric approach, the system is particularly well-suited for children, elderly individuals, or those with anxiety. The compact, mobile unit makes MRI scans possible at the bedside or in clinics where traditional fixed MRI systems are unavailable or impractical.
The Swoop® system is already showing promise in the field. It is currently being deployed in the NEURO PMR study, a Hyperfine-sponsored clinical trial evaluating the utility of portable MRI in neurology practices. Dr. Laszlo Mechtler, Medical Director at Dent Neurologic Institute and lead investigator of the study, praised the system’s performance: “This next-generation Swoop® delivers remarkable image quality while remaining compact and cost-effective. It offers neurology practices a powerful tool for on-site, same-day brain imaging that can improve both care and convenience.”
Dr. Jennifer Villa Frabizzio, a neuroradiologist at Jefferson Abington, called the system a “transformational advance” in portable imaging. “This redesign enhances patient comfort, improves workflow efficiency, and delivers image quality that meets the demands of clinical decision-making,” she said.
The launch of this next-generation system aligns with Hyperfine’s broader mission to make high-quality MRI more accessible globally. By leveraging artificial intelligence and engineering innovations, the company aims to bring advanced imaging capabilities to settings previously underserved by traditional MRI technology, including emergency departments, intensive care units, neurology clinics, and remote communities.
With FDA clearance secured, Hyperfine is preparing to roll out the new Swoop® system nationwide—paving the way for a new era in brain imaging that is faster, smarter, and more widely available than ever before.