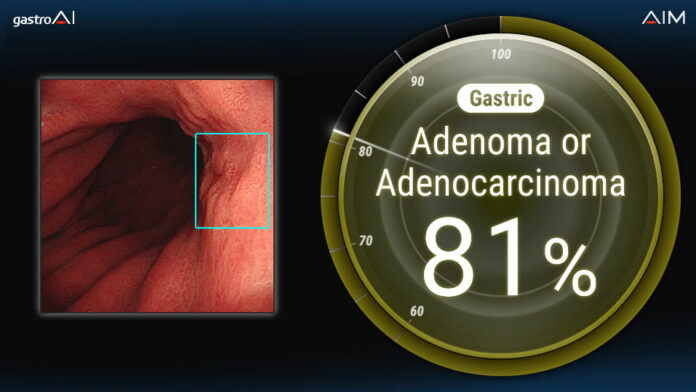
TOKYO — Japan-based medical startup AI Medical Service Inc. (AIM) has received regulatory approval from Thailand’s Food and Drug Administration for its AI-powered endoscopic diagnostic support software, the gastroAI-model G. The approval, granted on June 26, 2025, marks the first time an AI system for differentiating gastric lesions has been authorized for clinical use in Thailand.
The gastroAI-model G uses artificial intelligence to help physicians distinguish between neoplastic and non-neoplastic gastric lesions during endoscopic procedures. This approval positions the software as Thailand’s first AI-driven tool supporting upper gastrointestinal diagnoses with lesion differentiation capabilities.
Gastric cancer remains a significant health challenge in Thailand, where approximately 75% of diagnosed cases result in death—largely due to late-stage detection. While the global five-year survival rate for early-stage gastric cancer exceeds 90%, it drops below 50% when diagnosed at stage III or later. Early detection remains difficult, with studies suggesting that 4.5% to 25.8% of early-stage cases are missed during endoscopy.
“By delivering our gastric cancer diagnostic support AI leveraging Japan’s world-leading endoscopic medical technology to the clinical frontline in Thailand, we aim to address the anticipated shortage of endoscopy specialists and ultimately contribute to reducing the number of deaths from gastric cancer,” the company said in a statement.
Thailand’s rapidly aging population and improving life expectancy—currently around 76 years—are adding pressure on the healthcare system. With gastric cancer remaining among the top causes of cancer-related deaths, the adoption of AI-driven tools like gastroAI-model G is expected to enhance diagnostic accuracy and help address physician shortages.
AI Medical Service specializes in developing diagnostic support technologies using artificial intelligence and has positioned itself at the forefront of endoscopic innovation. The approval in Thailand represents a key step in the company’s global expansion strategy and its mission to reduce gastric cancer mortality through early, AI-assisted detection.