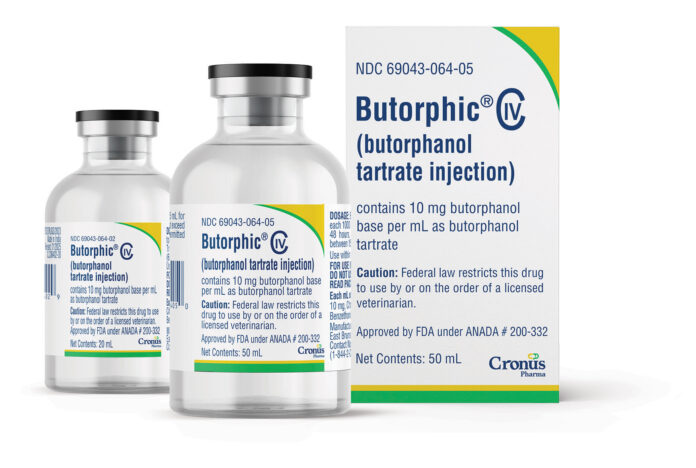
EAST BRUNSWICK, N.J. — Cronus Pharma LLC has announced the U.S. launch of Butorphic® (butorphanol tartrate) Sterile Injectable Solution, expanding its animal health portfolio to 25 products. The new medication is now available through national and regional distributors and is indicated for the relief of pain associated with colic and postpartum conditions in adult horses and yearlings.
“Butorphic is a vital analgesic for managing equine colic pain, and we are excited to grow our equine portfolio,” said Vimal Kavuru, Chairman and CEO of Cronus Pharma. “Importantly, Butorphic marks our fifth product launch this year, expanding our portfolio to twenty-five products with several more planned for 2025 and beyond.”
Approved by the U.S. Food and Drug Administration under ANADA #200-332, Butorphic is a generic equivalent to Torbugesic®, offering the same efficacy and safety profile. The product is available in 10 mg/mL concentrations and comes in 20 mL and 50 mL multi-dose vials. The 20 mL size is a unique offering that provides veterinarians with greater dosing flexibility.
The company noted that Butorphic strengthens its established sedative and anesthesia product line, which includes AnaSed® Equine (xylazine), Cropamezole™ (atipamezole), DexmedVet™ (dexmedetomidine), DetomiSed™ (detomidine), and ketamine injections.
“Butorphic’s launch reinforces our position among the animal health leaders in U.S. FDA ANADA approvals over the past several years,” said Edward Neugeboren, Chief Strategy Officer at Cronus. “It’s a testament to our team’s dedication to delivering high-quality, cost-effective pharmaceuticals to veterinarians and pet owners.”
Clinical studies show butorphanol tartrate is effective in alleviating abdominal pain caused by torsion, impaction, intussusception, spasmodic and tympanic colic, as well as postpartum pain in horses.