VALENCIA, Calif. — The U.S. Food and Drug Administration has approved SetPoint Medical’s novel implantable device for treating rheumatoid arthritis (RA), marking a major step forward in autoimmune disease care. The SetPoint System is the first FDA-approved neuroimmune modulation therapy for RA and offers a new option for patients who do not respond to or cannot tolerate current treatments such as biologic and targeted synthetic DMARDs.
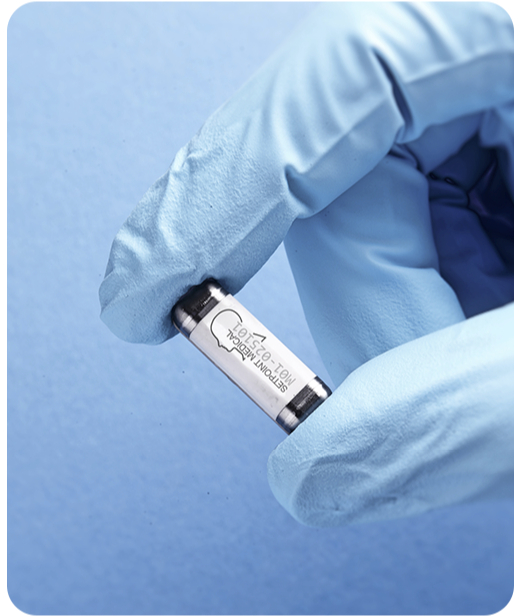
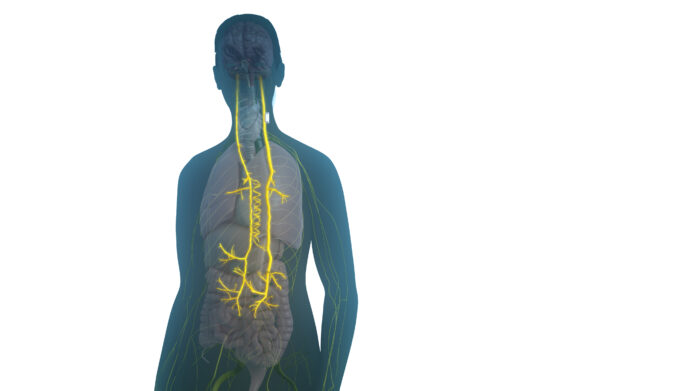
The device, implanted on the vagus nerve, delivers daily electrical stimulation to activate the body’s natural anti-inflammatory and immune-restorative responses. SetPoint Medical plans to launch the system in select U.S. cities this year, with a broader national rollout expected in early 2026.
“This approval represents a transformative milestone in the management of autoimmune diseases,” said SetPoint Medical CEO Murthy V. Simhambhatla, Ph.D. “We’re focused on expanding access to this innovative therapy to improve the health of people living with RA.”
RA affects more than 1.5 million Americans, with many patients discontinuing medications due to side effects or insufficient results. The SetPoint System aims to address those gaps without suppressing the immune system.
FDA approval was based on the 242-patient RESET-RA trial, a randomized, double-blind, sham-controlled study that showed the system met its primary efficacy endpoint, ACR20, at three months. Improvements continued through 12 months, with 75% of participants off biologic or targeted synthetic DMARDs at the one-year mark. The therapy was well-tolerated, with a 1.7% rate of serious adverse events and no reports of malignancies or serious infections linked to the device.
“This is a landmark study,” said Dr. John Tesser, the study’s national rheumatology principal investigator. “It confirms the potential of neuromodulation as a safe, effective alternative for patients with limited treatment options.”
Dr. Mark Richardson of Massachusetts General Hospital, the study’s surgical lead, noted the system can deliver therapy automatically for up to 10 years following a minimally invasive outpatient procedure.
SetPoint’s technology was previously granted FDA Breakthrough Device Designation and is being evaluated for use in other autoimmune diseases, including multiple sclerosis and Crohn’s disease.