BOCA RATON, Fla. — Medical device innovator SurGenTec has received expanded 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its flagship synthetic bone graft, OsteoFlo® HydroFiber™, marking a major regulatory milestone for the company. The newly approved indications now include use as a bone void filler in the treatment of tumors, cysts, trauma, and osteomyelitis—conditions that often present significant surgical challenges.
“This FDA clearance for OsteoFlo® HydroFiber™ is a major milestone for SurGenTec,” said Travis Greenhalgh, CEO and founder of the company. “Treating tumors and osteomyelitis presents significant challenges with limited options available. Our technology offers a powerful, autograft-equivalent solution that avoids the risks of autograft harvesting while still delivering exceptional outcomes.”
Initially cleared for spinal applications, including posterolateral fusions and interbody fusion cages, OsteoFlo HydroFiber is already recognized for its equivalence to traditional autograft. The latest clearance significantly broadens its clinical versatility, allowing it to be used across orthopedic and spinal surgeries involving complex bone defects.
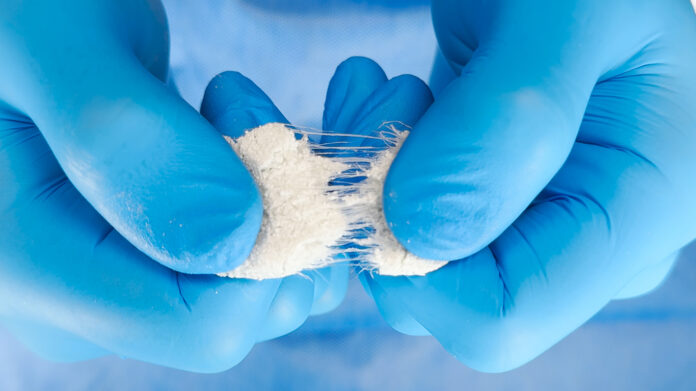
The product incorporates SurGenTec’s proprietary Web Interlace Technology, a design that suspends bone graft particles within hydrophilic fibers to improve cohesiveness, resist migration under irrigation, and ensure optimal flowability. This structure also facilitates absorption of saline, blood, or bone marrow aspirate, enhancing the biological environment needed for bone healing.
OsteoFlo HydroFiber can be used in conjunction with SurGenTec’s GraftGun®, a specialized delivery device that enables precise placement of the graft material into interbody cages and confined surgical spaces. This approach offers a significant improvement over conventional funnel-based methods, streamlining surgical workflow and improving graft delivery.
With its expanded indications, the synthetic graft is expected to reduce reliance on autografts and allografts, which are often associated with increased surgical risks and complications. SurGenTec says HydroFiber is now better positioned to support surgeons in treating patients with complex bone injuries and pathologies while improving outcomes and minimizing procedural invasiveness.