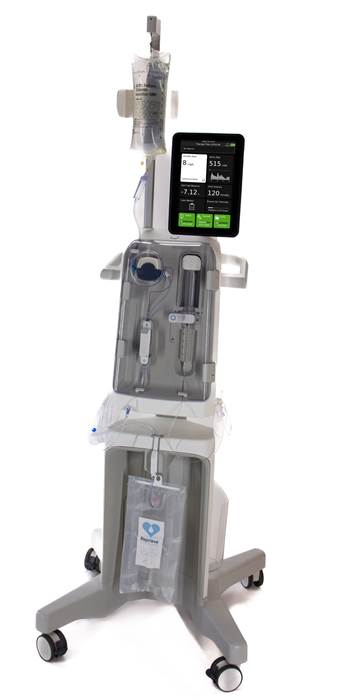
MILFORD, Mass. — Reprieve Cardiovascular, Inc., a clinical-stage company pioneering intelligent decongestion management therapy for acute decompensated heart failure (ADHF), has closed an oversubscribed $61 million Series B financing round and enrolled the first patient in its pivotal FASTR II clinical trial.
The funding round was led by Deerfield Management, with participation from Arboretum Ventures, Lightstone Ventures, Sante Ventures, Genesis Capital, Rex Health Ventures, Cadence Capital, and an undisclosed strategic investor. The financing combined equity investment with a debt facility to support the company’s growth. Proceeds will be used to accelerate the FASTR II trial and prepare for potential commercialization of the Reprieve System.
“We are pleased to close this latest financing round, which reflects the strong continued support of our existing investors, as well as the addition of new investors, who each bring unique strategic insights to Reprieve,” said Mark Pacyna, Chief Executive Officer of Reprieve Cardiovascular. “This capital ensures we are positioned to generate the clinical and economic evidence essential for regulatory approval and commercialization. We believe our personalized approach to decongestion management can enable better outcomes for both patients and healthcare systems around the world.”
David Neustaedter, Ph.D., venture partner at Deerfield Management and member of the Board of Directors, said, “We continue to be encouraged by the company’s clinical data and look forward to supporting their progress through this critical next stage of clinical and commercial development.”
Anita Watkins, managing director at Rex Health Ventures, the corporate venture fund of UNC Health, added, “Reprieve Cardiovascular is addressing a significant unmet need in today’s heart failure management paradigm, and the results from the FASTR pilot study underscore the potential of the Reprieve system to reimagine the standard of care for treating ADHF. We see unique opportunities to strategically partner with Reprieve to enhance and accelerate this innovative therapy.”
The FASTR II study follows the successful completion of the company’s FASTR randomized pilot study late last year, which met both primary efficacy and safety endpoints. The new trial will evaluate the Reprieve System’s ability to decongest patients compared to optimal diuretic therapy in up to 400 patients across the United States and Europe. Results are expected to support a future premarket approval submission in the U.S.
“Congrats to Dr. Ali Javaheri and the team at WashU Medicine on the first patient enrolled in the FASTR II study,” said Javed Butler, M.D., M.P.H., M.B.A., president of the Baylor Scott and White Research Institute, senior vice president for Baylor Scott and White Health, and global principal investigator of the FASTR II study. “On behalf of all the investigators in the study, we believe the Reprieve system represents a promising advancement for heart failure patients and physicians who currently face significant challenges with conventional approaches to decongestion. We look forward to rapid enrollment in the study and collectively contributing critical insights to help fundamentally change the standard of care for acute heart failure.”