MONTPELLIER, France– Womed®, a uterine health company focused on developing intrauterine treatments, announced today that the U.S. Food and Drug Administration (FDA) has granted PreMarket Approval (PMA) for the Womed Leaf®, a resorbable adhesion barrier for women undergoing hysteroscopic surgery to treat moderate to severe intrauterine adhesions (IUAs), also known as Asherman syndrome. This marks the first medical device approved in the United States specifically for this indication.
IUAs occur when scar tissue causes the uterine walls to bind together, often following procedures such as dilation and curettage or fibroid removal. The condition is a major cause of infertility, recurrent miscarriage, and chronic pelvic pain, and can affect 20 to 45 percent of women undergoing such procedures. Treatment has long been hindered by high recurrence rates, leaving many patients uncertain about their chances of conceiving.
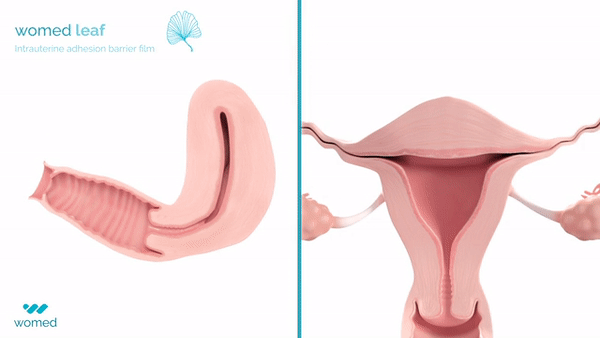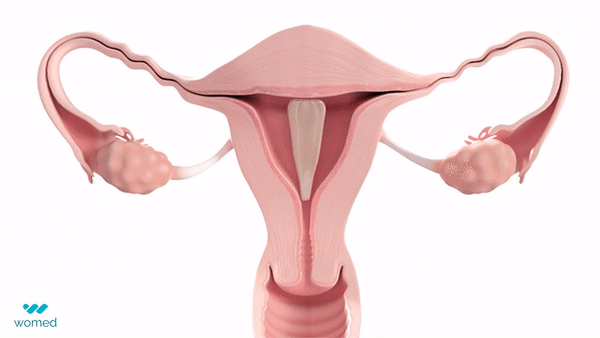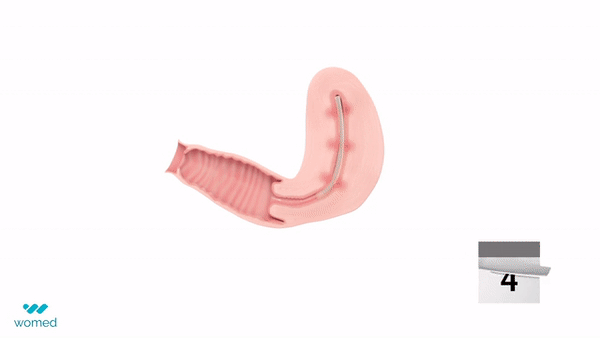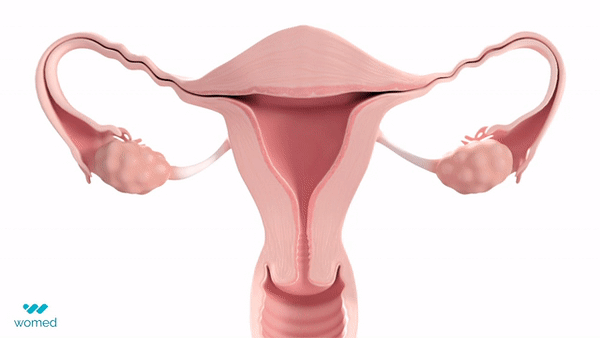
Womed Leaf® is designed to reduce the recurrence and severity of adhesions after surgery. The device is a thin, soft film made from Womed®’s proprietary polymer. Inserted at the end of an adhesiolysis procedure in a manner similar to an intrauterine device (IUD), it expands within the uterine cavity to keep the walls separated during healing and is later naturally and painlessly discharged.
The pivotal PREG2 randomized clinical trial enrolled 160 women with moderate to severe IUAs and demonstrated that Womed Leaf® significantly reduced adhesion severity compared with no prevention method, while maintaining an acceptable safety profile.
“Asherman syndrome prevents tens of thousands of women in the United States from becoming pregnant due to scar tissue in the uterine cavity. When adhesions are surgically removed, they often return because the uterine walls collapse together. Womed Leaf prevents that during the healing phase,” said Dr. Keith Isaacson, M.D., of Audubon Fertility and Ochsner Health System in New Orleans. “This is the first FDA-approved barrier for these patients and marks a significant improvement for their ultimate desired outcome.”
Gonzague Issenmann, co-founder and Chief Executive Officer of Womed®, added: “The meticulous review and inspections performed by the FDA for the PMA approval are a clear testimony to the rigorous work accomplished by our team. This milestone establishes Womed Leaf as the new standard for treating Asherman syndrome worldwide. We are thrilled to help American women in their fight against infertility when Womed Leaf becomes available in early 2026.”