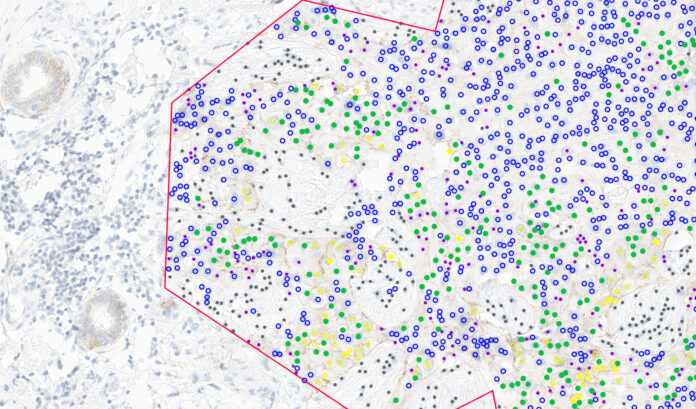
BOSTON– Ibex Medical Analytics, a leader in artificial intelligence (AI)-powered cancer diagnostics, announced it has received In Vitro Diagnostic Medical Devices Regulation (IVDR) certification for its HER2 breast cancer biomarker scoring solution. The fully automated “zero-click” decision support tool is designed to help pathologists increase accuracy and consistency in scoring HER2 immunohistochemistry (IHC), including HER2-low cases.
The solution, developed and validated in collaboration with AstraZeneca and Daiichi Sankyo, analyzes digitized images of HER2 IHC-stained breast cancer samples to detect invasive tumor cell staining patterns and classify HER2 expression into four standard scores (0, 1+, 2+, 3+) based on ASCO/CAP guidelines. It also assists in identifying ultralow HER2 expression, automates tumor detection and cell classification, and provides visualization of results to aid interpretation.
Two clinical studies, including one across 14 international cancer centers, demonstrated that pathologists using Ibex’s tool achieved higher accuracy and greater inter-observer consistency compared to manual scoring. According to study data, overall accuracy improved from 77.5 percent with manual review to 86.6 percent with AI support, while inter-observer agreement rose from 77.3 percent to 94.1 percent.
“AI can be a valuable decision support tool, aiding pathologists to improve HER2 scoring accuracy and align more closely with expert breast pathologists, as well as enhancing consistency among pathologists, particularly in challenging cases at the lower end of the HER2 expression spectrum,” said Dr. Elena Provenzano, lead breast histopathologist at Cambridge University Hospitals NHS Foundation Trust. “Ibex’s HER2 tool could be extremely valuable in today’s clinical landscape, where there is increasing pressure to identify low-expressing HER2 cases in a more reproducible and objective manner.”
Ibex said the IVDR certification followed a rigorous review process under EU regulations, underscoring confidence in the product’s safety and performance. The HER2 biomarker tool is part of Ibex Breast, an integrated AI solution already in use at leading pathology laboratories worldwide.
“Our AI-powered solutions are designed with pathologists, for pathologists, enabling them to leverage cutting-edge technology to make more confident diagnoses,” said Dr. Manuela Vecsler, Vice President of Clinical and Scientific Affairs at Ibex. “We are thrilled to see our HER2 IHC scoring solution’s high performance during IVDR clinical studies, with pathologists noting its ease of use, confidence boost, and smooth workflow integration—underscoring its real-world diagnostic impact. This certification reaffirms our commitment to providing the most accurate and reliable tools for pathologists, ultimately improving patient care.”