KUOPIO, Finland– Marginum, a Finnish medical technology company, announced that its flagship device, HIVEN, has been granted CE mark clearance following Medical Device Regulation (MDR) certification in just over 4.5 years—a record timeline for such approval.
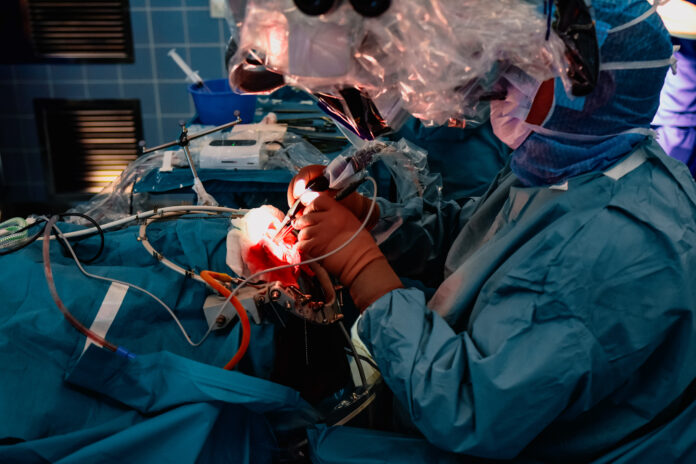
HIVEN is a first-of-its-kind intraoperative aspirate tissue monitoring (ATM) device designed to assist neurosurgeons with real-time margin assessment during tumor resections. By providing immediate feedback, the device helps surgeons distinguish tumorous tissue from healthy tissue without disrupting standard workflows. Marginum says the technology represents a new era of precision in cancer surgery.
“Achieving clearance for the CE mark is a pivotal step in bringing the HIVEN into clinical practice and improving outcomes for patients in Europe,” said Juho Leskinen, Chief Technology Officer and co-founder of Marginum. “This certificate reflects the hard work of our team and the strength of our scientific and clinical foundations.”
The device has been validated in Nordic hospitals, where it demonstrated the ability to detect fluorescent cancer tissue from aspirated samples during surgery. By improving visibility in areas where anatomical constraints, blood, or deep-seated locations limit detection, HIVEN provides neurosurgeons with critical insights that can reduce the likelihood of reoperations and improve patient outcomes.
“In glioma surgery, our ability to distinguish tumor from healthier tissues is limited by anatomical constraints, blood and compromised visibility, particularly in deep-seated areas. We wanted HIVEN to provide critical feedback beyond sensory limitations; you can consider it a sixth sense for tumor detection,” said Antti-Pekka Elomaa, M.D., Ph.D., a consultant neurosurgeon and Marginum co-founder.
Approved for fluorescence-guided neurosurgery of high-grade gliomas, HIVEN enhances surgical accuracy by enabling objective tissue detection in difficult-to-access areas. The company says this breakthrough technology could help minimize surgical complications, improve long-term patient outcomes, and lower healthcare costs by reducing the need for repeat procedures.