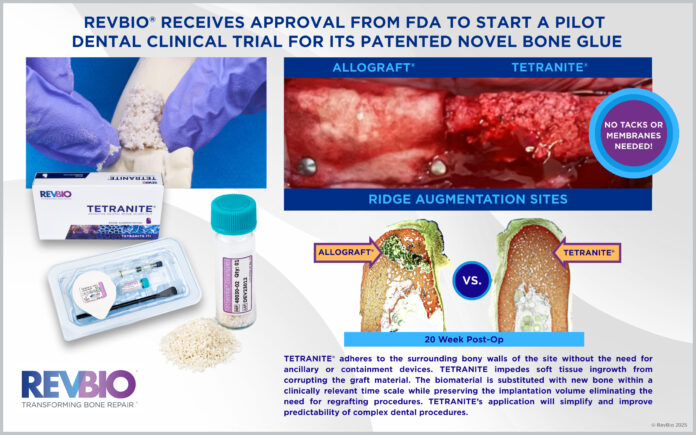
LOWELL, Mass. — RevBio Inc. said it has received approval from the U.S. Food and Drug Administration to begin a pilot clinical trial evaluating the safety and efficacy of TETRANITE, its regenerative bone adhesive, for use in dental ridge augmentation procedures.
The trial expands the range of clinical indications under development for TETRANITE and will assess whether the material can be used without ancillary containment devices such as membranes, meshes, tacks, or screws. RevBio said the adhesive is designed to bond directly to surrounding bony walls, prevent soft tissue ingrowth, and be gradually replaced by new bone while preserving implant volume.
“The ability for the product to adhere to the surrounding bony walls of a site that needs to be grafted is a fundamentally unique attribute of TETRANITE,” said Rahul Jadia, Ph.D., research and development manager of technology development at RevBio. “Equally impressive is the fact that the material is substituted with bone in a clinically relevant timescale without significant loss of volume or adhesive and mechanical strength, which will ultimately help accelerate the course of complex dental procedures.”
Dental ridge augmentation is commonly required for patients receiving dental implants who have experienced bone loss following tooth loss. RevBio said roughly 44 percent of implant patients present with missing teeth at the start of treatment and varying degrees of jawbone deterioration. Current procedures often rely on particulate bone graft materials that require fixation and containment devices to protect the graft during healing.
According to the company, more than 30 percent of ridge augmentation procedures fail to achieve the desired outcome using existing graft materials, requiring additional grafting surgeries that increase treatment time and cost. RevBio said TETRANITE is intended to simplify these procedures and improve predictability by eliminating the need for additional hardware and reducing the likelihood of regrafting.
Development of TETRANITE has been supported by multiple grants totaling $1.8 million from the Translational Resource Center, a research consortium funded by the National Institute of Dental and Craniofacial Research to advance tissue engineering and regenerative medicine technologies. RevBio also received a $2 million Direct to Phase II Small Business Innovation Research grant from the same institute to complete preclinical studies and advance the product into clinical development.
“The Translational Resource Center is excited about RevBio’s unique and compelling technology that addresses a critical unmet clinical need,” said David H. Kohn, Ph.D., director of the Translational Resource Center and a professor at the University of Michigan. “We are pleased to support RevBio and provide translational guidance that has helped move this product to regulatory approval and into first-in-human clinical trials.”