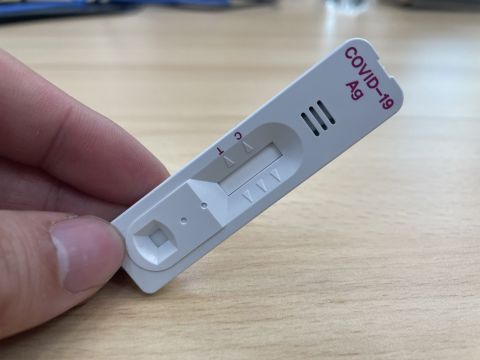
JURUPA VALLEY, Calif.– GenBody is proud to announce that the company’s visually readable COVID-19 antigen test kit has been issued an amendment to its original Emergency Use Authorization (EUA) by the U.S. Food and Drug Administration (FDA) for use with anterior nasal swabs.
GenBody sells and distributes direct nasopharyngeal and anterior nasal swab antigen tests intended for the qualitative detection of nucleocapsid protein antigen from SARS-CoV-2 to laboratories certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), 42 U.S.C. §263a, that meet the requirements to perform moderate, high, or waived complexity tests. The test is authorized for use at the point of care, i.e., in-patient care settings operating under a CLIA Certificate of Waiver, Certificate of Compliance, or Certificate of Accreditation. As the Biden-Harris Administration calls for more affordable and readily available rapid tests, GenBody has joined the lineup of relatively few manufacturers who are producing antigen test kits.
“The need for rapid test kits is especially strong now that we are seeing a rise in COVID-19 outbreaks and as the U.S. continues to open up,” said David Yoo, CEO of GenBody America. “We are excited to offer the GenBody COVID-19 Ag test to the U.S. and we are looking forward to bringing additional product releases to market.”
GenBody was awarded a National Institutes of Health (NIH) Rapid Acceleration of Diagnostics (RADx℠) initiative award of $10 million in support of U.S. production of GenBody’s innovative point of care tests that will increase the testing capacity in the U.S.

