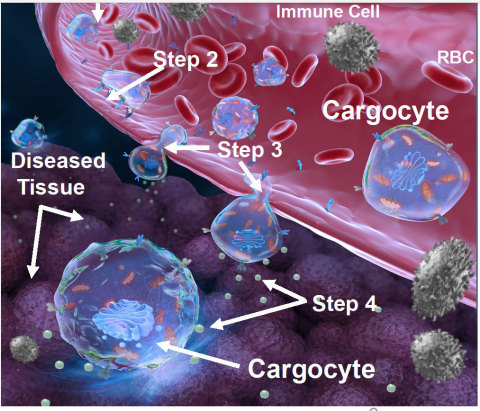
SAN DIEGO– Cytonus Therapeutics Inc., a US-based biotechnology company developing an innovative drug-delivery platform technology called Cargocytes, today announced the publication of a peer reviewed research paper in the high impact journal, Nature Biomedical Engineering. The study showed that Cargocytes, which are genetically engineered with a GPS-like cellular navigation system, can selectively target disease tissue within hours after being administered and produce bioactive therapeutics at high levels for several days, thus allowing for a new modality in treating a wide range of difficult to treat diseases that require precision drug delivery.
Results from the preclinical study provide further support for the innovative therapeutic potential of the Cargocyte technology platform. In addition, the Cargocyte’s unique ability to penetrate deep into target tissues and act as an in vivo “therapeutic factory” to produce bioactive immunomodulators at high concentrations within that targeted disease tissue is a major differentiation from simple drug-delivery approaches such as nanoparticles, red blood cells and exosome-based technologies. The groundbreaking study was led by Cytonus Therapeutics co-founder and Chief Science Officer, Richard Klemke Ph.D., Professor of Pathology at the University of California San Diego and Moores Cancer Center.
“These preclinical animal studies highlight the fact that the Cargocyte platform establishes a highly differentiated new category of drug-delivery and expands our view of precision drug delivery,” said Klemke. “Our Cargocyte product represents a true vertical move in the field of drug delivery and medicine with real potential to impact multiple disease indications.”
“What’s remarkable about this study is that it demonstrates the critical role drug localization plays in the delicate balance between efficacy and toxicity of a therapeutic. We are all looking for better outcomes with fewer side effects and we now have an elegant way to do this,” said Remo Moomiaie-Qajar, M.D., co-founder and CEO of Cytonus Therapeutics. “For the first time, therapeutics can be produced and secreted at the sites of disease in a controlled and safe manner. We believe this will allow for greater precision in delivering drugs and provide better efficacy and safety outcomes for patients.”

