CLEVELAND– Medical device and technology company Lazurite Holdings LLC today announced it has submitted a 510(k) premarket notification to the U.S. Food and Drug Administration (FDA) for its ArthroFree™ wireless camera system for minimally invasive surgery, and that the submission has been accepted for review. The company also announced that early-stage healthcare investment platform AngelMD named it one of the best startup companies of 2021.
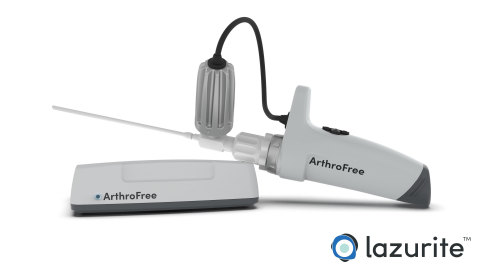
The ArthroFree system is expected to be the world’s first FDA-approved fully wireless, minimally invasive camera system for the operating room. The modular system incorporates the company’s proprietary low-heat, high-intensity Meridiem™ light engine technology along with advanced camera, battery, and wireless transmission technologies. The ArthroFree system is designed to deliver improved operating room productivity, patient safety, and economic value through cost-savings, energy efficiency, and reduced setup/breakdown times. The system is designed to be fully drop-in compatible with current operating room technology.
“This FDA submission marks an important milestone in our commitment to bring an advanced wireless surgical camera to market,” said Eugene Malinskiy, chief executive officer and co-founder of Lazurite. “During the nearly 50 years since the introduction of the first fiber-optic scope, the industry has seen only a slow evolution in image quality, monitors, and usability. The ArthroFree system is designed to untether the camera from the surgical tower, which is expected to make it safer and easier to use, while reducing OR setup and turnover times and delivering exceptional light and image quality during minimally invasive surgery.”
“The ArthroFree system promises to usher in a new era of wireless, minimally invasive camera systems for the operating room,” said Mark Froimson, MD, Chair of the Board of Managers of Lazurite. “Our FDA approval process doesn’t require any human or animal trials, so there is good reason to believe that we will receive FDA clearance for market launch by mid-2022. Based on the response from surgeons we’ve met at industry trade shows and on visits to leading medical centers across the country, we believe the system will be poised to redefine industry expectations for the use of surgical cameras.”