EWING, N.J.– Orcosa Inc. (“Orcosa” or the “Company”), a life sciences company modernizing the way medicines are taken through its next generation drug delivery technology – the Rapid Infusion Technology (RITe™) Platform – today announced its proud support of the positive results from NYU Langone Health’s (“NYU Langone”) multicenter Phase 1/2 clinical trial utilizing Orcosa’s lead product candidate, ORAVEXX™ (cannabidiol, CBD). The trial, which was conducted jointly at NYU Langone and Baptist Health/Jacksonville Orthopaedic Institute and led by Principal Investigator Dr. Michael J. Alaia of NYU Langone, sought to evaluate safety and impact on post-operative pain, patient satisfaction and opioid consumption following arthroscopic rotator cuff repair in 99 patients.
The findings presented by Dr. Alaia at the Annual Meeting of the American Academy of Orthopaedic Surgeons demonstrated that cannabidiol (CBD) administered utilizing the RITe™ Platform effectively reduces pain after shoulder surgery with no safety concerns. Highlights of the results include:
- On the first day after surgery, patients receiving ORAVEXX™ experienced on average 23% less pain as measured by the Visual Analog Scale (VAS) pain score compared to patients receiving the placebo.
- On both the first and second days after surgery, patients receiving ORAVEXX™ reported 22% – 25% greater satisfaction with pain control compared to those receiving placebo.
- Patients receiving 50 mg of CBD reported lower pain and higher satisfaction with pain control compared to patients receiving placebo or 25 mg of CBD.
- The ORAVEXX™ group outperformed the control group at all time points.
- No major side effects were reported.
“We are grateful for the patients and investigators whose participation in this study evaluating CBD as a pain management therapy may lead to improved treatment options for patients,” said Mark Ridall, Founder and Chief Executive Officer of Orcosa. “We are thrilled with the positive results of this trial, which lays the foundation for several upcoming Phase 2 studies in the treatment of acute and chronic pain utilizing ORAVEXX™. This is a critical step in validating the strength and viability of our technology platform, and we are grateful to NYU Langone and Baptist Health/Jacksonville Orthopaedic Institute for their partnership.”
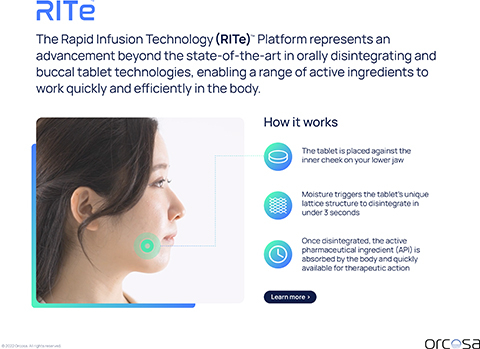
Orcosa is the inventor, developer, and exclusive owner of the RITe™ Platform – a fast acting, easy-to-take tablet engineered to enhance drug absorption. The tablet is designed to rapidly infuse an active ingredient through the tissue in the mouth and enable therapeutic effects quickly and efficiently. Based on the unique manner of administration, RITe™ tablets have the ability act faster and provide enhanced bioavailability over current formulations while reducing the potential for unwanted side effects for certain compounds. All RITe™ products are manufactured at the Company’s state-of-the-art cGMP-compliant facility in New Jersey.
ORAVEXX™ is a non-addictive proprietary cannabidiol composition that utilizes the RITe™ Platform to treat pain and has the potential to provide a safe, alternative treatment option to opioids and NSAIDs. Historically, research on the effects of CBD has been limited by a variety of issues including inaccurate dosing, low bioavailability, difficult administration, and a lack of GMP-manufactured products with matching placebos with which to conduct clinical research. ORAVEXX™ is being developed to address these issues associated with unreliable and inefficient orally delivered CBD and provide researchers the opportunity to evaluate CBD more accurately in clinical trials.
Along with Dr. Alaia, study authors were Eoghan T. Hurley, Kinjal Vasavada, Danielle H. Markus, Briana Britton, Guillem Gonzalez-Lomas, MD, Andrew S. Rokito, MD, and Laith M. Jazrawi, MD, from NYU Langone Health, and Kevin Kaplan, MD, of Baptist Health in Jacksonville, Florida.