CHICAGO– Mesh Suture, Inc., d.b.a. MSi, today announced that it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for DURAMESH™ non-absorbable polypropylene mesh suture, a pioneering medical device for the surgical closure of soft tissues including muscles, fascia, tendons, and ligaments.
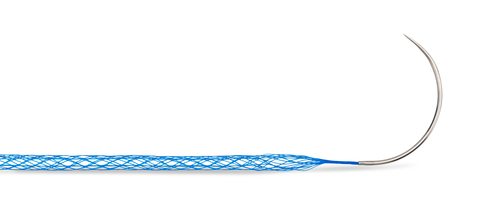
DURAMESH™ is a first-of-its-kind medical device, combining the desirable principle of implant incorporation in a mesh repair with the placement precision of a suture for soft tissue repairs.
DURAMESH™ aims to mitigate the intractable problem of surgical failure due to suture pull-through. The sharp leading edge of a conventional suture can slice through otherwise intact tissue, potentially leading to dehiscence, hernia formation, and poor tendon function. DURAMESH™’s novel architecture flattens at the suture-tissue interface to resist pull-through. DURAMESH™’s open-walled hollow design also allows tissue ingrowth for implant incorporation with no capsule formation. In a porcine study, DURAMESH™ had numerically fewer loose sutures and hernias in comparison to conventional suture.1
“DURAMESH™ offers the perfect combination of strength and simplicity in a surgical repair,” says Dr. Gregory Dumanian, Chief Medical Officer at MSi. “It combines the handling characteristics of traditional suture with a mesh polyfilament design. We are excited to bring this innovative technology to our surgeon colleagues and to their patients who need it most. By designing DURAMESH™ to address the surgical complication of suture pull-through, we expect to see sizeable improvements in patient outcomes.”