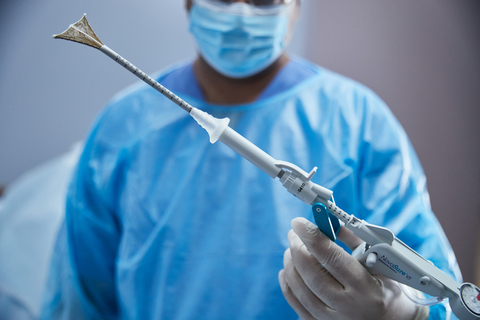
MARLBOROUGH, Mass.– Hologic Inc. (Nasdaq: HOLX), a global leader in women’s health, has announced approval of the NovaSure® V5 global endometrial ablation (GEA) device in Canada and Europe. This innovative new version incorporates enhanced features designed to treat a wide range of cervical and uterine anatomies. It builds on a world-class track record for NovaSure, including 3 million patients who have benefited from the device.
“Since its launch over 20 years ago, we have constantly listened to our customers’ feedback, which has driven continuous design improvements for NovaSure,” said Jan Verstreken, Group President, International at Hologic. “This has enabled us to develop our technology to further meet their needs and those of the women they treat, ultimately delivering better outcomes for these women.”
NovaSure V5 has an updated cervical seal, featuring EndoForm™ technology, designed to increase the sealing surface and accommodate a range of cervical canals and anatomical variability.1,2,3 The device’s AccuSheath™ markings are designed to improve the accuracy and confidence of seating and fundal placement.4 Additionally, NovaSure V5 is equipped with SureClear™ technology, Hologic’s unique fluid removal system, which provides integrated suction through the array by constant tissue contact while simultaneously removing ablation byproducts such as vapor and fluid.5
“I welcome the new features on the NovaSure V5. These will support both inexperienced and more established users in performing effective, more efficient and accurate procedures,” said Dr. Philippe Y. Laberge, M.D., FRCSC, ACGE based at the CHU de Québec-Université Laval in Québec, Canada. “The addition of markings to the shaft will enable users to correctly place the device before initiating a procedure. The cervical seal is a significant improvement as there is less chance of leaks, allowing the completion of pre-procedure checks and the procedure itself.”
The NovaSure device enables an endometrial ablation procedure for the treatment of abnormal uterine bleeding, with 97% patient satisfaction and 87% of patients avoiding a hysterectomy at 10 years.6,7
References
1 NovaSure V5 cervical seal drawing, FAB-18821
2 Baggish, MS, Karram, MM. Atlas of Pelvic Anatomy and Gynecologic Surgery. 4th edition. Elsevier 2016. ISBN: 978-0323225526. Chapter 44, Page 493
3 Luo, J et al. Quantitative analyses of variability in normal vaginal shape and dimension on MR images. Int. Urogynecol (2016) 27:1087-1095
4 NovaSure V5 Designs Verification Results, VER-10513
5 NovaSure V5 Instructions for Use, MAN-07653-001
6 Baskett TF, Clough H, Scott TA. NovaSure bipolar radiofrequency endometrial ablation: report of 200 cases. Journal of Obstetricians and Gynecologists Canada. 2005;27(5):473–476.
7 Herman M, Penninx J, Mol B, Bongers M. Ten-year follow-up of a randomised controlled trial comparing bipolar endometrial ablation with balloon ablation for heavy menstrual bleeding. BJOG 2013;120:966–970