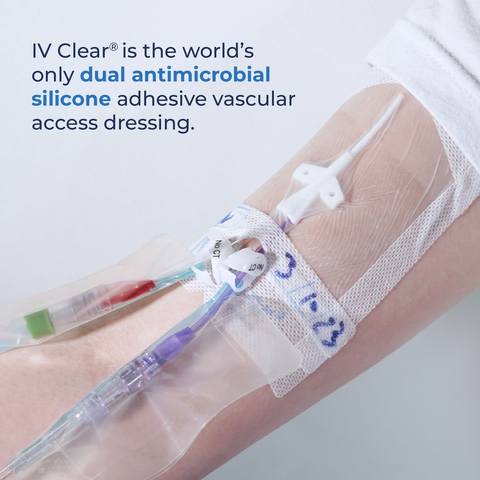
MISSISSAUGA, Ontario– Covalon Technologies Ltd. (the “Company” or “Covalon”) (TSXV: COV; OTCQX: CVALF), an advanced medical technologies company, today announced that its IV Clear® line of dual antimicrobial silicone dressings has been recommended by physicians at a Top 10 U.S. Children’s Hospital in Colorado, which is also an EB (“Epidermolysis Bullosa”) Center of Excellence, as a novel solution to effectively secure and protect vascular access insertion sites on patients with fragile skin, including those with EB.
EB, sometimes known as “Butterfly Skin”, is a little-known skin disorder with a prevalence of 1 in 20,000 births. There is no cure to the debilitating connective tissue disorder that causes extremely fragile skin prone to blisters and tears from minor friction. EB patients often require surgical procedures, and securement of intravenous (IV) catheters and peripheral nerve catheters on EB patients present major challenges to anesthesiologists.
In a statement, Kim Strupp MD, FAAP and Norah Janosy, MD, said, “As pediatric anesthesiology physicians who care for patients with Epidermolysis Bullosa (EB) routinely when they need surgical interventions or procedural care, IV Clear® has been transformative in our practice. We are now able to place this transparent, occlusive dressing over intravenous, central and nerve block catheters without damaging our EB patients’ fragile skin. Having a clear, transparent, occlusive dressing decreases the risk of infection, increases the visibility of the catheter, and has improved our care and patient and family satisfaction.”
The recommendation follows research completed by physicians at Children’s Hospital of Colorado (“CHCO”), where they observed how the use of IV Clear® in EB patients improved the safety of indwelling catheter placement and securement without causing injury. The findings were presented by Drs. Mancone, Strupp, Janosy, and Brooks Peterson at the International Anesthesia Research Society meeting in Denver, Colorado on April 14, 2023. To date, IV Clear® dressings have been used on approximately 20 EB patients at CHCO without incidence of skin damage or other adverse events. One patient who spent several months in the hospital underwent several consecutive dressing applications without incident.
“At Covalon, our mission has been driven by compassion and a commitment to improving patient care,” said Ron Hebert, SVP Marketing of Covalon. “Healing should never be hindered by setbacks, trauma, or tears caused by dressings. IV Clear® is designed to ensure that every step of a patient’s journey is one of comfort and compassion.”
Traditional securement dressings made with acrylic cause substantial injury upon removal. Covalon’s IV Clear® contains atraumatic silicone adhesive and its gentle, dual antimicrobial properties make it ideal for use on patients at risk of skin injury.