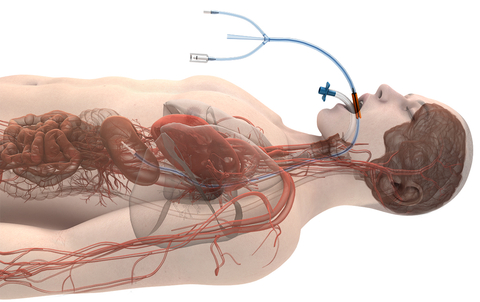
CHICAGO– Attune Medical®, a pioneer in the field of esophageal temperature management with its ensoETM® device, is providing significant support for the upcoming AF Symposium as part of the company’s corporate mission. AF Symposium, scheduled for February 1-3 in Boston, Massachusetts, gathers the world’s leading electrophysiologists to share, in a highly interactive environment, the latest advances in the treatment of atrial fibrillation.
As part of its mission to support education and enhance patient outcomes, Attune Medical is the primary corporate sponsor of the AF Symposium EP Fellows’ Scholarship Program and the dedicated Interactive Educational Program for fellows on Saturday, February 3 from 4-8 p.m. The AF Symposium Fellows’ Program offers a unique opportunity to engage with leaders in Cardiac Electrophysiology during this exclusive four-hour session.
A highlight of the Symposium, with the company’s proactive esophageal cooling as a focal point, will be Spotlight Session 1 (New and Emerging Technologies in Arrhythmia Management) on Thursday, February 1 at 8:30 a.m. At this session, Dr. Dinesh Sharma will present “Proactive Esophageal Cooling – Data Update and Overview,” focusing on the benefits of esophageal cooling beyond safety alone, including shorter procedure times, reduced fluoroscopy requirements, greater hospital savings, and improved long-term freedom from arrhythmia.
In addition, proactive esophageal cooling will be discussed in several poster presentations including:
- Multi-Site Analysis of the Impact on Throughput Associated with Proactive Esophageal Cooling;
- Cost Analysis of Left Atrial Ablation Procedures Using Active Esophageal Cooling – Influence of Procedure Time;
- Low Continuity Index Using Active Esophageal Cooling During Radiofrequency Ablation;
- Acute and Long-Term Procedural Effects with Proactive Esophageal Cooling During Radiofrequency Ablation;
- Reduction of Atrioesophageal Fistula Rates After Adoption of Proactive Esophageal Cooling During Atrial Fibrillation Ablation – Further Statistical Analysis.
The company will also feature prominently on the conference show floor at booth 217, providing an opportunity for hands-on interaction with the ensoETM.
Attune received De Novo marketing authorization from the US FDA for use of its ensoETM to reduce the likelihood of ablation-related esophageal injury resulting from radiofrequency cardiac ablation procedures in September 2023, and has distributed over 60,000 devices to more than 200 hospitals and electrophysiology labs.
Dr. Sharma, Section Head of Electrophysiology for the Rooney Heart Institute and the Fritz and Kathy Friday Endowed Chair of Electrophysiology at NCH Healthcare System in Naples, Florida, commented, “I’ve been using proactive esophageal cooling for a year now, and appreciate the patient safety benefits, as well as the additional improvements it offers to procedural efficiency.”