SYDNEY– Novotech, a globally recognized full-service clinical research organization (CRO) and scientific advisory company, has released its newest preclinical report exploring the evolving landscape of small interfering RNA (siRNA) therapeutics. This report provides insights for biotech and pharmaceutical companies leveraging RNA-based precision medicine to develop next-generation treatments for oncology, metabolic disorders, and rare genetic diseases.
Unlocking the Potential of siRNA for Drug Development
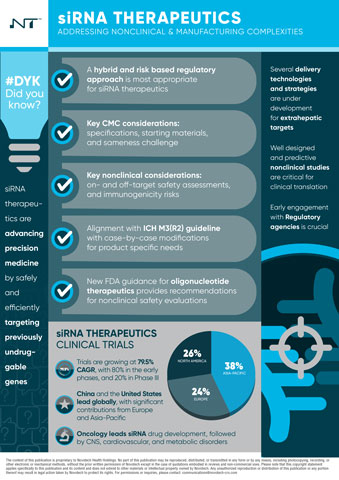
siRNA therapeutics represent a paradigm shift in targeted drug development by enabling precise gene silencing of disease-causing genes. With the FDA approval of multiple siRNA-based drugs, including ONPATTRO™ (Patisiran) and Inclisiran, the field is witnessing rapid advancements in delivery platforms, stability, and tissue-specific targeting. Novotech’s report outlines the current research and regulatory landscape, highlighting how siRNA technologies are expanding the druggable genome, addressing previously undruggable targets.
Key highlights
- Clinical and Regulatory Landscape: Overview of the six FDA-approved siRNA therapies and their expanding indications in cardiovascular, hepatic, and rare genetic diseases.
- Advancements in siRNA Delivery: Innovations in lipid nanoparticles (LNPs), GalNAc conjugates, and polymeric nanoparticles enhancing targeted tissue delivery and minimizing immune-related challenges
- Expanding Applications in Oncology: siRNA’s role in silencing oncogenes, overcoming drug resistance, and targeting tumor microenvironments, positioning it as a promising alternative to traditional cancer treatments.
- Clinical Trial Growth & Future Outlook: Analysis of the 150+ industry-sponsored siRNA clinical trials conducted between 2020 and 2024, with a 79.5% CAGR reflecting significant industry investment.