Santa Clara, Calif.– Ancora Heart, Inc. announced today that it has reached a critical enrollment milestone in its pivotal CORCINCH-HF clinical trial, which is evaluating the safety and effectiveness of the company’s investigational AccuCinch® Transcatheter Left Ventricular Restoration System in patients with heart failure with reduced ejection fraction (HFrEF). With 250 patients now randomized, the company plans to use six-month follow-up data from this cohort to support a Premarket Approval (PMA) submission to the U.S. Food and Drug Administration (FDA). Total trial enrollment will continue toward a target of 400 patients.
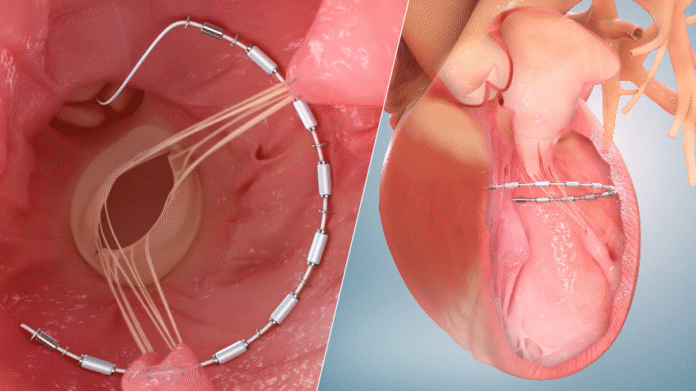
The AccuCinch System is a first-of-its-kind transcatheter therapy designed to reduce the size of the enlarged left ventricle, relieve ventricular wall stress, and reinforce the heart wall in patients suffering from HFrEF. It is currently the only completely transcatheter procedure under investigation for this purpose. The device received Breakthrough Device Designation from the FDA in 2022, reflecting its potential to address a critical unmet need in the treatment of heart failure.
Ancora Heart CEO Jeff Closs described the milestone as a major achievement in advancing heart failure treatment. He emphasized the collaborative effort behind the trial, praising the dedication of physicians and research teams across participating clinical sites. “Reaching this milestone is an incredible accomplishment in heart failure research,” Closs said. “We look forward to building on this momentum as we move toward full enrollment.”
The CORCINCH-HF study targets patients with symptomatic HFrEF, a condition affecting approximately 3 million Americans. Despite advancements in medical therapy, many patients continue to experience progressive symptoms and frequent hospitalizations.
Dr. Ulrich Jorde, global co-principal investigator of the trial and section head of heart failure and cardiac transplantation at Montefiore Health System in New York, called the enrollment milestone a significant step in determining whether the AccuCinch System can offer patients a meaningful improvement in quality of life and clinical outcomes.
Dr. Mark Reisman, co-principal investigator and director of structural heart disease at NewYork-Presbyterian/Weill Cornell Medical Center, explained that the device is designed to reverse the remodeling of the left ventricle, which occurs in advanced heart failure. He said the trial is intended not only to assess the safety of the device and the procedure but also to determine whether patients may benefit from improved cardiac function, reduced hospitalizations, and potentially longer survival.
Early results from clinical studies of the AccuCinch System were presented in 2023 and published in the Journal of Cardiac Failure, laying the foundation for the ongoing pivotal evaluation. With continued enrollment and data collection underway, Ancora Heart is moving closer to what could become a new treatment option for patients whose lives are limited by progressive heart failure.