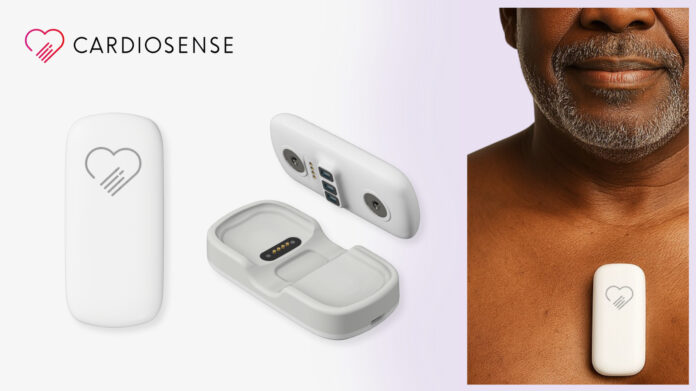
CHICAGO — Cardiosense has received 510(k) clearance from the U.S. Food and Drug Administration for its CardioTag™ device, a wearable sensor designed to provide a detailed, noninvasive assessment of heart function by capturing multiple types of physiological signals simultaneously.
The CardioTag combines electrocardiogram (ECG), photoplethysmogram (PPG), and seismocardiogram (SCG) technologies into a single patch, enabling clinicians to analyze electrical activity, blood flow, and mechanical motion of the heart in real time. This multimodal approach is intended to offer more nuanced insights into cardiac health, including hemodynamic status and pressure metrics typically obtained through more invasive procedures.
According to the company, the device is now authorized for measuring SCG, ECG, and PPG signals as well as heart rate and pulse rate. SCG, in particular, detects micro-vibrations in the chest wall caused by the heart’s mechanical action. When analyzed alongside ECG and PPG data, the device can assess cardiac timing intervals such as left ventricular ejection time—a key indicator of heart efficiency.
“This milestone represents a significant advancement in noninvasive cardiac care,” said Amit Gupta, Cardiosense co-founder and CEO. “Until now, cardiac monitoring has largely focused on rhythm analysis through ECG. With CardioTag, we’re adding new physiological dimensions that enhance the ability to evaluate cardiac performance.”
The company has also developed an artificial intelligence algorithm designed to estimate pulmonary capillary wedge pressure (PCWP), a critical metric for managing heart failure patients. The algorithm, which previously received Breakthrough Device designation from the FDA, has demonstrated accuracy comparable to implantable sensors in clinical trials. Cardiosense plans to pair the PCWP software with CardioTag once it secures regulatory approval.
“This clearance opens the door to more accessible, pressure-guided treatment,” said Andrew Carek, co-founder and CTO. “The rich data collected by CardioTag provides the foundation for developing AI models that can support real-time decision-making in cardiac care.”
Built on years of interdisciplinary research spanning engineering, cardiology, and data science, the CardioTag device is positioned to play a central role in remote and in-clinic heart monitoring. Co-founder and Chief Scientific Officer Omer Inan noted that the clearance marks a “deeply meaningful milestone” in the company’s long-standing effort to deliver reliable, noninvasive hemodynamic insights into everyday clinical practice. (Source: IANS)