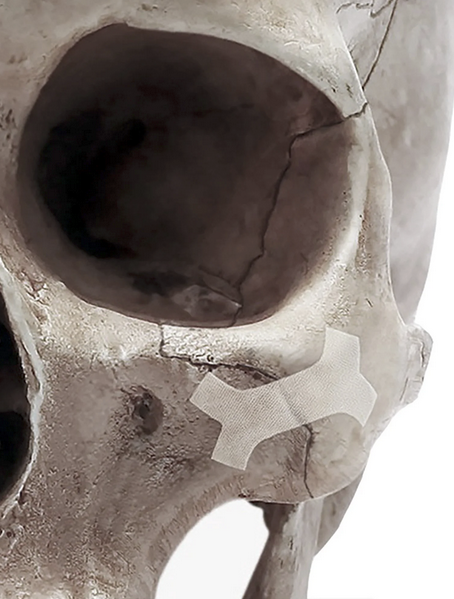
TORONTO– Cohesys Inc., a Toronto-based developer of resorbable fixation technologies, announced the first clinical use of its investigational BoneTape device in patients with non-load-bearing craniofacial fractures. The procedures, completed as planned, mark the initial human application of the company’s drill- and screw-free fixation system.
“Reaching this milestone is significant for Cohesys as we evaluate BoneTape’s potential to offer a simpler, patient-friendly approach to fracture repair,” said Dr. Michael Floros, CEO of Cohesys. “The data from this study will inform our regulatory strategy and commercialization pathway.”
The study is being led by Dr. Jeffrey A. Fialkov, head of the Division of Plastic and Reconstructive Surgery at Sunnybrook Health Sciences Centre and associate professor at the University of Toronto. “BoneTape’s hardware-free approach is intended to simplify surgery and may improve the recovery experience for patients,” Fialkov said.
BoneTape is applied using a handheld applicator that secures the resorbable device to bone without drilling or screws. Cohesys said the study will assess whether the design can reduce device-related complications and eliminate the need for hardware-removal procedures.
Device- and procedure-related risks, including infection, non-union, and re-operation, will be monitored during the study. “The completion of these first cases with BoneTape reflects the dedication of our team and our clinical partners,” said Dr. Janaina Bortolatto, vice president of clinical operations at Cohesys. “We are focused on gathering the clinical data needed to evaluate BoneTape for potential future use.”