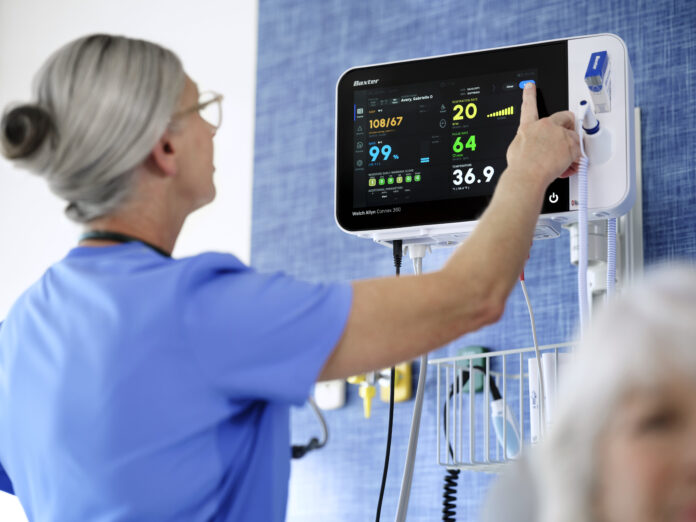
DEERFIELD, Ill.– Baxter International Inc. (NYSE: BAX), a global medtech leader, today announced the launch of the Welch Allyn Connex 360 Vital Signs Monitor, its next-generation patient monitoring device. The system is designed to enhance clinical workflows and improve patient safety with advanced connectivity, security features, and customizable configurations that support earlier detection of patient deterioration while allowing for future device upgrades.
“There is nothing more important than keeping patients safe, especially when time is of the essence,” said Julie Foster, President of Front Line Care at Baxter. “Care teams rely on vital signs monitoring to detect changes in their patients’ conditions and make informed decisions. With Connex 360, we are advancing patient monitoring to provide care teams with timely insights at the bedside, so they can make the most of every moment with their patients.”
Connex 360 captures vital signs for adult, pediatric, and neonatal patients, including blood pressure, temperature, pulse rate, respiration rate, and blood oxygen levels. Clinicians can obtain a full set of vitals in less than one minute, with the data automatically uploaded to electronic medical records, helping reduce manual entry errors and supporting informed clinical decisions. The secure transfer of information is powered by Baxter’s cloud-based DeviceBridge platform, which enables interoperability across hospital IT systems and Baxter’s ecosystem of connected devices.
The monitor includes enhanced security features such as end-to-end encryption and certificate-based authentication to safeguard patient and facility information. It also offers Baxter’s largest and most responsive color touchscreen for easier navigation and the ability to view more patient data on a single screen. Customizable Early Warning Score protocols help alert clinicians to signs of patient deterioration, and configurable monitoring modes support the individual needs of patients. In addition, streamlined software upgrades for clinical, operational, and cybersecurity improvements reduce the burden on hospital IT teams and allow the device to expand its functionality over time.
The launch builds on Baxter’s strengthened position in patient monitoring following its acquisition of Hillrom and the Welch Allyn portfolio. Welch Allyn has a longstanding reputation for delivering innovative monitoring solutions across hospitals and outpatient care. The Connex 360 monitor has received FDA 510(k) clearance and is now available for hospitals and health systems in the United States.