LOWELL, Mass.– RevBio, Inc. announced it has received a $2.2 million Phase II Small Business Innovation Research (SBIR) grant from the National Institute on Aging (NIA), part of the National Institutes of Health (NIH). The two-year grant (1R44AG097243-01) builds upon the company’s earlier Phase I project (1R43AG079741-01A1) and will fund continued preclinical testing of RevBio’s TETRANITE® biomaterial for the treatment of vertebral compression fractures. This work represents a key step toward U.S. Food & Drug Administration approval to initiate human clinical trials.
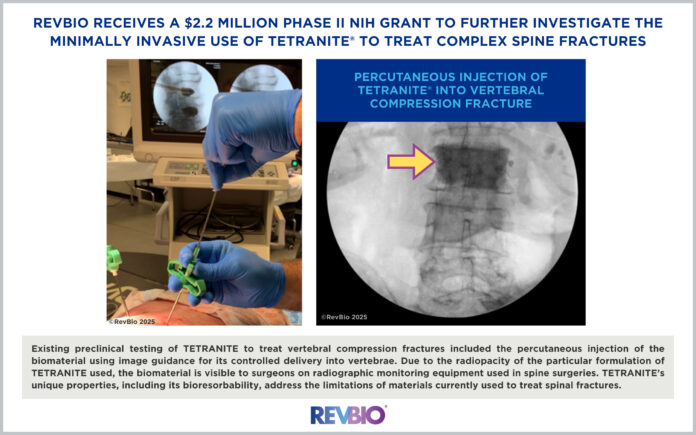
The research aims to demonstrate improvements over the current standard of care for spinal fracture treatment by using TETRANITE in a minimally invasive procedure. Existing preclinical testing has shown that TETRANITE can be percutaneously injected into vertebrae under image guidance, providing controlled delivery and visualization on radiographic monitoring equipment due to its radiopacity. The biomaterial’s bioresorbable and osteoconductive properties allow it to integrate with bone and gradually be replaced by natural bone tissue — a significant advantage over existing materials.
“The adhesive and structural features of this biomaterial, combined with the fact that it is osteoconductive and ultimately replaced by bone, make it an excellent candidate for an improved vertebroplasty procedure,” said Kevin T. Foley, M.D., Professor of Neurosurgery, Orthopedic Surgery, and Biomedical Engineering at the University of Tennessee Health Science Center, and Chairman of the Semmes Murphey Clinic. Dr. Foley is an internationally recognized leader in minimally invasive spine surgery techniques.
In the United States, approximately 850,000 vertebral fractures occur each year, most commonly in older adults. Nearly one in five individuals over the age of 70 experience these fractures due to osteoporosis. Traditional surgical treatments — vertebroplasty and kyphoplasty — involve the injection of polymethylmethacrylate (PMMA) cement to stabilize fractured vertebrae. While these procedures can relieve pain and restore mobility, PMMA has significant drawbacks, including thermal damage to bone, lack of biodegradability, and rigidity that can lead to secondary fractures in adjacent vertebrae. Cement leakage occurs in up to 75% of cases and can cause severe complications such as pulmonary embolisms in roughly 23% of patients. These risks have led to a 70% decline in such procedures since 2009, leaving many elderly patients without effective relief for chronic pain.
“Having been involved in the Phase I preclinical testing of this product, I’ve seen firsthand how TETRANITE’s game-changing properties address the limitations that led to the decline in PMMA use for spinal fractures,” said Eric Woodard, M.D., Chief of Neurosurgery at New England Baptist Hospital.
“Kyphoplasty, developed by Kyphon, Inc., was extremely successful in treating chronic pain, leading to Kyphon’s $3.9 billion acquisition by Medtronic,” added Jonathan Slotkin, M.D., practicing neurosurgeon and General Partner at Scrub Capital, which recently invested in RevBio. “The biomimetic characteristics of TETRANITE provide a much better product profile than PMMA and could establish it as the new standard of care — and potentially a blockbuster product — for treating chronic pain associated with spine fractures.”
RevBio’s work with TETRANITE continues to demonstrate the promise of advanced biomaterials in orthopedic and neurosurgical innovation, supporting the company’s mission to develop safer, more effective solutions for complex bone injuries.