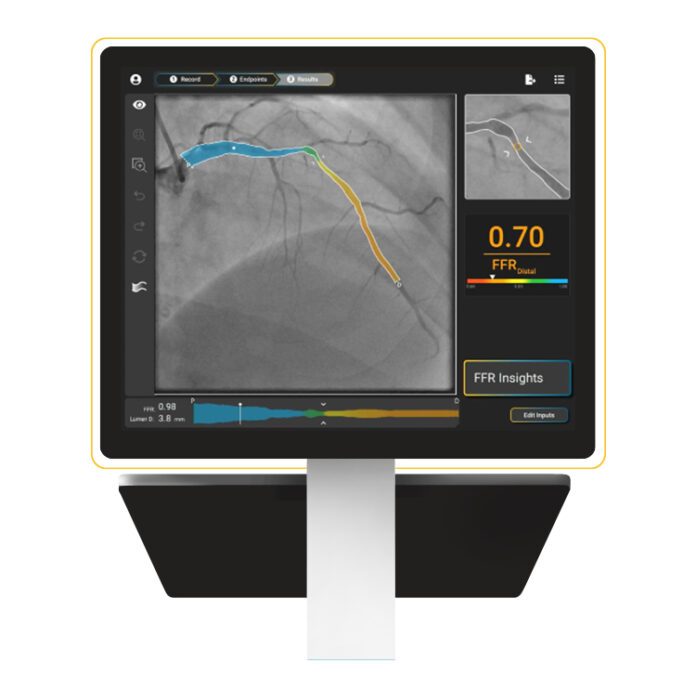
BEDFORD, Mass. – SpectraWAVE, Inc., a medical imaging company advancing technologies to improve the treatment of coronary artery disease (CAD), announced that the U.S. Food and Drug Administration (FDA) has granted 510(k) clearance for its X1-FFR system. The AI-enabled, wire-free, drug-free software provides physiology results from a single angiogram in real time and will be available as an add-on to SpectraWAVE’s HyperVue Imaging System. Together, the two products form the first and only U.S. platform that combines angiogram-derived physiology with intravascular imaging.
X1-FFR delivers physiology data from a single angiogram via a direct real-time feed, eliminating the need for network-based DICOM file transfers. The software uses AI-driven workflows, including automated vessel segmentation and frame suggestion, to simplify and accelerate fractional flow reserve (FFR) assessments.
“Physiology is essential for PCI outcomes, and replacing invasive pressure wires with non-invasive angiogram-derived physiology is better and safer for our patients,” said Michael C. Kim, M.D., Director of the Cardiac Cath Labs at Lenox Hill Hospital and Director of Interventional Cardiology for the Western Region of Northwell Health. “SpectraWAVE’s X1-FFR represents the next stage in angio-derived physiology, requiring only one view per vessel to derive accurate FFR values. In our clinical experience, results can be achieved in less than one minute. We believe this technology will see widespread adoption across the U.S. and internationally.”
“HyperVue is already valued as a comprehensive, easy-to-use intravascular imaging product,” said Eman Namati, Ph.D., Chief Executive Officer of SpectraWAVE. “With the addition of X1, HyperVue becomes a unique central hub for the cath lab, combining angiogram-derived physiology and intravascular imaging on one platform. By leveraging AI, X1 enables fast, accurate, and streamlined procedures. This milestone marks our fifth FDA clearance in just over two years and reflects our continued commitment to advancing patient outcomes through innovation.”
Approximately 2.5 million diagnostic angiograms and one million percutaneous coronary interventions (PCI) are performed each year in U.S. cardiac catheterization labs. Traditionally, physiology assessments require invasive pressure wires and drugs such as adenosine to guide treatment decisions, while intravascular imaging optimizes PCI outcomes. HyperVue with X1-FFR provides a wire-free, drug-free alternative that integrates both functions into a single, efficient workflow.
SpectraWAVE will showcase HyperVue with X1-FFR at TCT 2025 in San Francisco, with live demonstrations at Booth 2027 and an Academic Satellite Program featuring case presentations on Monday, October 27, from 3:45 to 4:45 PM at the Moscone Convention Center.